Which of the following facts given is not correct/
(I) Bond length order :
(II) have same bond order of
(III) Bond order can assume any value including zero upto four
(IV) and have same bond order for X - O bond (where X is central atom)
1. I, II & III
2. I & IV
3. II & IV
4. I & II

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
and are converted to monocations and respectively, which is wrong statements:
1. In , the bond weakens
2. In , the bond order increases
3. In , the paramagnetism decreases
4. becomes diamagnetic

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
In which of the following transformations, the bond order has increased and the magnetic behavior has changed?

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
and both are covalent compounds but is polar whereas is non-polar. This is because :
1. Nitrogen atom is smaller than boron atom
2. N-F bond is more polar than B-F bond
3. NF is pyramidal whereas BF is planar triangular
4. BF is electron deficient whereas NF is not
How many sp and sp-hybridised carbon atoms are present respectively in the following compound ?
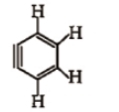
1. 4, 2
2. 6, 0
3. 3, 3
4. 5, 1
Which molecule does not exist ?

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
0.01 mole of is completely neutralized by 0.56 gram of KOH hence :
1. x = 3 and given acid is dibasic
2. x = 2 and given acid is monobasic
3. x = 3 and given acid is monobasic
4. x = 4 and given acid forms three series of salt

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
The shape of is :
1. Trigonal planar
2. Pyramidal
3. Bent T-shape
4. See-saw

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Which is the following pairs of species have identical shapes ?

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.


