SN1 mechanism for the reaction,
R-X+KOHROH+KX follow:
(1) carbocation mechanism
(2) carbanion mechanism
(3) free radical mechanism
(4) either of these
The elimination reaction among the following is:
1. CH3CH3+Cl2CH3CH2Cl+ HCl
2. CH3Cl+ KOH(aq.) CH3OH +KCl
3. CH2=CH2 +Br2 CH2BrCH2Br
4. C2H5Br+ KOH(alc.) C2H4 + KBr+H2O
The SN2 mechanism for R-X + KOH (aq) R-OH + KX follows with:
(1) 100% inversion
(2) 50% inversion
(3) 40% inversion
(4) 30% inversion
Which of the following is an example of the elimination reaction?
1. Chlorination of methane
2. Dehydration of ethanol
3. Nitration of benzene
4. Hydroxylation of ethylene
The formation of cyanohydrin from a ketone is an example of:
(1) electrophilic addition
(2) nucleophilic addition
(3) nucleophilic substitution
(4) electrophilic substitution
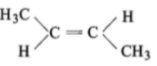
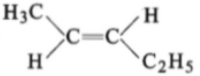
The compound which reacts with HBr obeying Markownikoff's rule is:
1.
2. 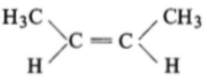
3. 
4. 
If X is halogen the correct order for SN2 reactivity is-
1. R2CHX > R3CX > RCH2X
2. RCH2X > R3CX > R2CHX
3. RCH2X > R2CHX > R3X
4. R3CX > R2CHX > RCH2X
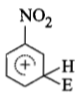
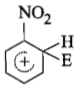
The electrophile. E attacks the benzene ring to generate the intermediate - complex. Of the following which - complex is of lowest energy?
1. 
2. 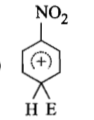
3. 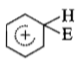
4. 
Consider the following bromides:
the correct order of SN1 reactivity is:
1. A>B>C
2. B>C>A
3. B>A>C
4. C>B>A
KI in acetone, undergoes SN2 reaction with each of P, Q, R and S, the rates of the reaction vary as:
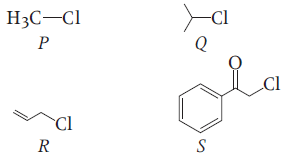
1. P > Q > R > S
2. S > P > R > Q
3. P > R > Q > S
4. R > P > S > Q







