Choose the incorrect assertion regarding binding energy per nucleon:
1. Binding energy per nucleon is practically constant for nuclei with mass numbers between 30 and 170.
2. Binding energy per nucleon is maximum for (equal to 8.75 meV).
3. Binding energy per nucleon for is lower compared to .
4. Higher the binding energy per nucleon, the more unstable is the nucleus.

To unlock all the explanations of 14 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 14 chapters you need to be enrolled in MasterClass Course.
Which of these is Q-value of decay of into ?
( represent masses of Na-atom, Ne-atom, and electron respectively)
1.
2.
3.
4.

To unlock all the explanations of 14 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 14 chapters you need to be enrolled in MasterClass Course.
A sample of radioactive material has mass m, decay constant and molecular weight M. Avogadro constant = . The initial activity of the sample is:
1.
2.
3.
4.

To unlock all the explanations of 14 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 14 chapters you need to be enrolled in MasterClass Course.
Consider the information given. Nuclear mass of = 40.962278 u, nuclear mass of = 39.962591 u, mass of neutron = 1.00866 u. Neutron separation energy for is:
1. 7.458 MeV
2. 8.358 MeV
3. 9.568 MeV
4. 10.008 MeV

To unlock all the explanations of 14 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 14 chapters you need to be enrolled in MasterClass Course.
Two radioactive materials have decay constant respectively. If initially, they have the same number of nuclei, then the ratio of the number of nuclei will be after a time:
1.
2.
3.
4.

To unlock all the explanations of 14 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 14 chapters you need to be enrolled in MasterClass Course.
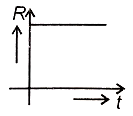
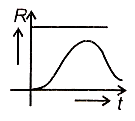
A radioactive nucleus X decays to a stable nucleus Y. Then, the graph of the rate of formation R of Y against time will be:
1. 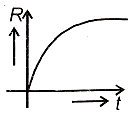 2.
2. 
3. 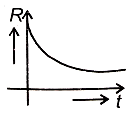 4.
4. 

To unlock all the explanations of 14 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 14 chapters you need to be enrolled in MasterClass Course.
A radioactive sample has a half-life of 30 minutes. At 3 PM, its decay rate was measured as 120,000 counts/s. What will be the decay rate at 5 PM?
1. 120,000 counts/second
2. 60,000 counts/second
3. 30,000 counts/second
4. 7,500 counts/second

To unlock all the explanations of 14 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 14 chapters you need to be enrolled in MasterClass Course.
Consider the following nuclear reactions:
Then :
1. X and Y are both protons.
2. X and Y are both neutrons.
3. X is a proton and Y is a neutron
4. X is a neutron and Y is a proton

To unlock all the explanations of 14 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 14 chapters you need to be enrolled in MasterClass Course.
A nucleus of lead emits two-electron followed by an alpha particle. The resulting nucleus will have:
1. 82 protons and 128 neutrons
2. 80 protons and 130 neutrons
3. 82 protons and 130 neutrons
4. 78 protons and 134 neutrons

To unlock all the explanations of 14 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 14 chapters you need to be enrolled in MasterClass Course.
Consider the following statements:
(I) All isotopes of elements have the same number of neutrons.
(II) Only one isotope of an element can be stable and non-radioactive.
(Ill) All elements have isotopes.
(IV) All isotopes of Carbon can form chemical compounds with Oxygen-16.
The correct option regarding an isotope is-
1. III and IV only
2. II, III, and IV only
3. I, II, and III only
4. I, III, and IV only

To unlock all the explanations of 14 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 14 chapters you need to be enrolled in MasterClass Course.


