In the preparation of compounds of Xe, Bartlett had taken as a base compound. This is because
1. Both O2 and Xe have same size.
2. Both O2 and Xe have same electron gain enthalpy.
3. Both O2 and Xe have almost same ionisation enthalpy.
4. Both Xe and O2 are gases.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Reduction potentials of some ions are given below. Arrange them in decreasing order of oxidising power.
Ion
Reduction potential
1.
2.
3.
4.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Match the items of Columns I and II and mark the correct option.
Column I Column II
(A) H2SO4 (i) Highest electron gain enthalpy
(B) CCl3NO2 (ii) Chalcogen
(C) Cl2 (iii) Tear gas
(D) Sulphur (iv) Storage batteries
(1) A(iv) B(iii) C(i) D(ii)
(2) A(iii) B(iv) C(i) D(ii)
(3) A(iv) B(i) C(ii) D(iii)
(4) A(ii) B(i) C(iii) D(iv)

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Match the items of Columns I and II and mark the correct option.
Column I Column II
(A) Its partial hydrolysis does not (i) He
change oxidation state of central atom
(B) It is used in modern diving apparatus (ii) XeF6
(C) It is used to provide inert atmosphere (iii) XeF4
for filling electrical bulbs
(D) Its central atom is in sp3d2 hybridisation (iv) Ar
1. A(i) B(iv) C(ii) D(iii)
2. A(i) B(ii) C(iii) D(iv)
3. A(ii) B(i) C(iv) D(iii)
4. A(i) B(iii) C(ii) D(iv)

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
The chemical formula of 'laughing gas' is
1. NO
2.
3.
4.
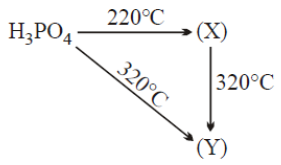
1. (X) = Phosphorous acid, (Y) = Metaphosphoric acid
2. (X) = Pyrophosphoric acid, (Y) = Metaphosphoric acid
3. (X) = Metaphosphoric acid, (Y) = Pyrophosphoric acid
4. (X) = Metaphosphoric acid, (Y) = Phosphene gas
Which one of the following compounds on strong heating evolves ammonia gas ?
1.
2.
3.
4.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Match List - I with List - II
|
List-I |
Chemical reaction |
List-II |
Names of process |
|
I. |
(a) |
Contact process |
|
|
II. |
(b) |
Ostwald's process |
|
|
III. |
(c) |
Deacon's process |
|
|
IV. |
(d) |
Haber's process |
1. I-a, II-b, III-d, IV-c
2. I-b, II-c, III-a, IV-d
3. I-a, II-d, III-c, IV-b
4. I-a, II-c, III-b, IV-d

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Ammonia can be dried by
1. conc.
2.
3. CaO
4. anhydrous
There is no S-S bond in
1.
2.
3.
4.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.


