The chemical formula of 'laughing gas' is
1. NO
2.
3.
4.
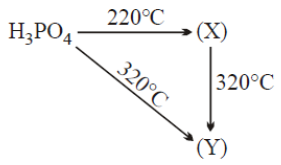
1. (X) = Phosphorous acid, (Y) = Metaphosphoric acid
2. (X) = Pyrophosphoric acid, (Y) = Metaphosphoric acid
3. (X) = Metaphosphoric acid, (Y) = Pyrophosphoric acid
4. (X) = Metaphosphoric acid, (Y) = Phosphene gas
Which one of the following compounds on strong heating evolves ammonia gas ?
1.
2.
3.
4.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Match List - I with List - II
|
List-I |
Chemical reaction |
List-II |
Names of process |
|
I. |
(a) |
Contact process |
|
|
II. |
(b) |
Ostwald's process |
|
|
III. |
(c) |
Deacon's process |
|
|
IV. |
(d) |
Haber's process |
1. I-a, II-b, III-d, IV-c
2. I-b, II-c, III-a, IV-d
3. I-a, II-d, III-c, IV-b
4. I-a, II-c, III-b, IV-d

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Ammonia can be dried by
1. conc.
2.
3. CaO
4. anhydrous
There is no S-S bond in
1.
2.
3.
4.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Which of the following leaves no residue on heating ?
1.
2.
3.
4.
Which amongst the following reactions cannot be used for the preparation of the halogen acid?
1.
2.
3.
4.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Which of the following pair has bleaching property ?
1.
2.
3.
4.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Nitrogen is liberated by the thermal decomposition of only
1.
2.
3.
4. All the three

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.


