is a stronger acid than HCIO. The correct statement is
1. ion is more stabilized than
2. ion has higher hydration energy than
3. is better solvated in water than HClO
4. In , H is attached to Cl, while in HClO it is attached to O
Number of single bonds and P-S double bonds (P=S) in are respectively
1. 10, 6
2. 16, 0
3. 14, 2
4. 12, 4
If a dilute solution of aqueous is saturated with then the product formed is
1.
2.
3.
4.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
The reaction that does not produce nitrogen is
1. Heating
2. Excess of
3. Heating of
4. Heating of

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
With respect to halogens, four statements are given below
(I) The bond dissociation energies for halogens are in the order:
(II) The only oxidation state is —1
(III) The amount of energy required for the excitation of electrons to the first excited state decreases progressively as we move from F to l
(IV) They form species in their aqueous solutions (X = halogen)
The correct statements are
1. I, II, IV
2. I, III, IV
3. II, III, IV
4. I, III

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Bleaching powder contains a salt of an oxoacid as one of its components. The anhydride of that acid is
1.
2.
3.
4.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Which of the following molecular structures is not possible?
1.
2.
3.
4.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Which of the following salt/s of exists?
I. II. III.
1. I and II only
2. I, II, and III
3. II and III only
4. III only

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
The gas which liberates bromine from a solution of KBr is
1.
2.
3.
4. Hl
Which one of the following is not a valid structure for dinitrogen oxide?
l. 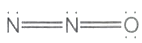
ll. 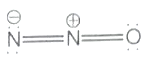
lll. 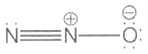
lV. 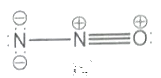
1. l
2. ll
3. lll
4. lV

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.


