With respect to halogens, four statements are given below
(I) The bond dissociation energies for halogens are in the order:
(II) The only oxidation state is —1
(III) The amount of energy required for the excitation of electrons to the first excited state decreases progressively as we move from F to l
(IV) They form species in their aqueous solutions (X = halogen)
The correct statements are
1. I, II, IV
2. I, III, IV
3. II, III, IV
4. I, III

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Bleaching powder contains a salt of an oxoacid as one of its components. The anhydride of that acid is
1.
2.
3.
4.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Which of the following molecular structures is not possible?
1.
2.
3.
4.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Which of the following salt/s of exists?
I. II. III.
1. I and II only
2. I, II, and III
3. II and III only
4. III only

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
The gas which liberates bromine from a solution of KBr is
1.
2.
3.
4. Hl
Which one of the following is not a valid structure for dinitrogen oxide?
l. 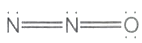
ll. 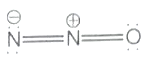
lll. 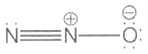
lV. 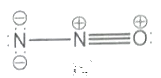
1. l
2. ll
3. lll
4. lV

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
The molecule which is NOT hydrolyzed by water at 25C is
1.
2.
3.
4.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
The number of P-H bond(s) in , , and , respectively, is
1. 2, 0, 1
2. 1, 1, 1
3. 2, 0, 0
4. 2, 1, 0

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
The complete hydrolysis of \(\mathrm{XeF}_6\)
| 1. | \(\mathrm{XeO}_2 \mathrm{~F}_2\) | 2. | \(\mathrm{XeOF}_4\) |
| 3. | \(\mathrm{XeO}_3\) | 4. | \(\mathrm{XeO}_2\) |

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Phosphorous reacts with chlorine gas to give a colorless liquid. which fumes in moist air to produce HCl and
1.
2.
3.
4.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.


