A system is taken from state \(A\) to state \(B\) along two different paths \(1\) and \(2.\) If the heat absorbed and work done by the system along these two paths are \(Q_1,Q_2\) and \(W_1,W_2\) respectively, then
1. \(Q_1=Q_2\)
2. \(W_1=W_2\)
3. \(Q_1-W_1=Q_2-W_2\)
4. \(Q_1+W_1=Q_2+W_2\)
A given mass of gas expands from state \(A\) to state \(B\) by three paths \(1, 2~\text{and}~3\), as shown in the figure. If \(W_1, W_2~\text{and}~W_3\) respectively be the work done by the gas along the three paths, then:

| 1. | \(W_1 >W_2>W_3\) | 2. | \(W_1<W_2<W_3\) |
| 3. | \(W_1 =W_2=W_3\) | 4. | \(W_1 <W_2=W_3\) |
An ideal gas changes from state 'a' to state 'b' as shown in figure. What is the work done by the gas in the process?

1. zero
2. positive
3. negative
4. infinite
A carnot engine having an efficiency of th of heat engine, is used as a refrigerator. If then work done on the system is 10 J, the amount of energy absorbed from the reservoir at lower temperature is:
1. 1 J
2. 90 J
3. 99 J
4. 100 J
The temperature inside a refrigerator is and the room temperature is . The amount of heat delivered to the room for each joule of electrical energy consumed ideally will be:
1.
2.
3.
4.
A refrigerator works between 4°C and 30°C. It is required to remove 600 calories of heat every second in order to keep the temperature of the refrigerated space constant. The power required is (Take, 1 cal = 4.2 Joules)
1. 23.65 W
2. 236.5 W
3. 2365 W
4. 2.365 W
Figure below shows two paths that may be taken by a gas to go from a state \(A\) to a state \(C.\) In process \(AB,\) \(400\text{ J}\) of heat is added to the system and in process \(BC,\) \(100\text{ J}\) of heat is added to the system. The heat absorbed by the system in the process \(AC\) will be-
1. \(380\text{ J}\)
2. \(500\text{ J}\)
3. \(460\text{ J}\)
4. \(300\text{ J}\)
One mole of an ideal diatomic gas undergoes a transition from A to B along a path AB as shown in the figure.
The change in internal energy of the gas during the transition is
1. 20 kJ
2. -20 kJ
3. 20 J
4. -12 kJ
The coefficient of performance of a refrigerator is 5. If the temperature inside freezer is -20°C, the temperature of the surroundings to which it rejects heat is -
1. 31°C
2. 41°C
3. 11°C
4. 21°C
A thermodynamic system is taken through the cycle ABCD as shown in figure. Heat rejected by the gas during the cycle is
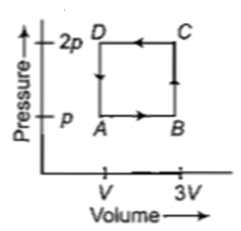
1. 2 pV
2. 4 pV
3.
4. pV








