-D-glucose and -D glucose have a specific rotation of +112 and +19 respectively. In aqueous solution the rotation becomes +52. This process is called:
1. inversion
2. racemisation
3. mutarotation
4. embolism
A racemic mixture is formed by mixing two:
1. Isomeric compounds
2. Chiral compounds
3. Meso compounds
4. Enantiomers
A compound of molecular formula C7H16 shows optical isomerism, the compound will be
1. 2,3-dimethyl pentane
2. 2,2-dimethyl butane
3. 2-methyl hexane
4. None of the above
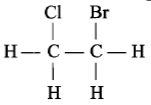
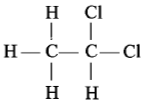
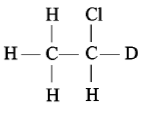
Which of the following has an asymmetric carbon atom?
1. 
2. 
3. 
4. 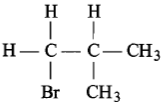
Stereoisomers (geometrical or optical) that are neither superimposable nor a mirror images to each other are called:
1. Enantiomers
2. Monomers
3. Tautomers
4. Diastereomers
Diastereomers can be separated by:
1. Recrystallization
2. simple distillation
3. electrophoresis
4. all of these
Which of the following pairs of carbon skeletons is an example of isomerism?
| 1. |  |
| 2. |
|
| 3. |  |
| 4. |  |
The number of isomeric structures for C2H7N would be:
1. 4
2. 3
3. 2
4. 1
Number of isomers possible for C4H8 are-
1. 4
2. 3
3. 2
4. 6
The dihedral angle between the hydrogen atoms of two methyl groups in staggered conformation of ethane is:
1. 120°
2. 180°
3. 90°
4. 60°







