CH3CH2ClXYZ
In the above reaction sequence, Z is
1. CH3CH2CH2NHCOCH3
2. CH3CH2CH2NH2
3. CH3CH2CH2CONHCH3
4. CH3CH2CH2CONHCOCH3
Predict the product,

| 1. |  |
| 2. |  |
| 3. |  |
| 4. |  |
Intermediates formed during the reaction of RCONH2 with Br2 and KOH are -
1. RCONHBr and RNCO
2. RNHCOBr and RNCO
3. RNHBr and RCONHBr
4. RCONBr2
In a reaction of aniline a colored product C was obtained.

The structure of C would be
| 1. |  |
| 2. |  |
| 3. |  |
| 4. |  |

Product Q in the above reaction is
| 1. |  |
| 2. |  |
| 3. |  |
| 4. |  |
The final product C in the reaction mentioned below is:
 \(\xrightarrow[]{Ac_2O}\ A\ \xrightarrow[CH_3COOH]{Br_2}\ B\ \xrightarrow[H^+]{H_2O}\ C\)
\(\xrightarrow[]{Ac_2O}\ A\ \xrightarrow[CH_3COOH]{Br_2}\ B\ \xrightarrow[H^+]{H_2O}\ C\)
| 1. |  |
2. |  |
| 3. |  |
4. |  |
Aniline when diazotised in cold and then treated with dimethylaniline, gives a coloured product. Its structure would be :
1. 
2. 
3. 
4. 
Aniline in a set of the following reactions yielded a coloured product Y.
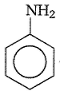
The structure of Y would be
| 1. |  |
| 2. |  |
| 3. |  |
| 4. |  |
What is the end product in the following sequences of operations?
Acetamide
1. CH3NH2
2. C2H5NH2
3. CH3CN
4. CH3COONH4
Name the end product in the following series of reactions,
CH3COOH ABC:
1. CH4
2. CH3OH
3. acetonitrile
4. ammonium acetate






