Enzymes work at optimum temperature. Over a range of 0 to 400C, what would happen to the rate of enzyme controlled reactions for every 100C rise in temperature?
1.The rate doubles itself
2.Decreases by half
3.No effect
4.First increases then decreases
Which of the following is true about carbonic anhydrase?
1.It accelerates the rate of formation of carbonic acid by 10 million times.
2.In its absence only 200 molecules of carbonic acid is formed in an hour.
3.It forms 600,000 molecules of carbonic acid in one second.
4.All of these
Find the correct statement
1.Catalysed reactions proceed at rates higher than that of uncatalysed ones.
2.In skeletal muscle under anaerobic conditions lactic acid is formed from pyruvic acid.
3.In yeast during fermentation ethanol is formed from pyruvic acid.
4.All of these
Which of the following is correct?
1.The chemical or metabolic conversion refers to a reaction.
2.The chemical which is converted into a product is called a substrate.
3.Proteins with three dimensional structures including an active site is called enzyme.
4.All of these
Which of the following describes the given graph correctly?
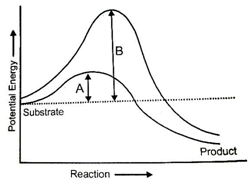
1.Endothermic reaction with energy A in presence of enzyme and B in absence of enzyme
2.Exothermic reaction with energy A in presence of enzyme and B in absence of enzyme
3.Endothermic reaction with energy A in absence of enzyme and B in presence of enzyme
4.Exothermic reaction with energy A in absence of enzyme and B in presence of enzyme
The figure given below shows the conversion of a substrate into product by an enzyme. In which one of the four options (1 - 4) the components of reaction labelled as A, B, C and D are identified correctly ?
|
A |
B |
C |
D |
|
|
1. |
Potential energy |
Transition state |
Activation Energy with enzyme |
Activation energy with- out enzyme |
|
2. |
Transition State |
Potential Energy |
Activation Energy with-out enzyme |
Activation energy with enzyme |
|
3. |
Activation energy with-out enzyme |
Transition state |
Activation energy with enzyme |
Potential energy |
|
4. |
Activation energy with enzyme |
Transition state |
Activation energy with-out enzyme |
Potential energy |
Which of the following is correct?
1.The ES complex formation is a transient phenomenon
2.The structure of the substrate gets transformed into the structure of product.
3.All the structures formed between substrate and product are called intermediates.
4.All of these
Which of the following is not true for the graph?
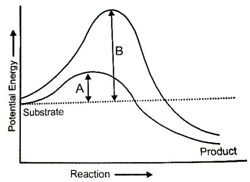
| 1. | The reaction is exothermic |
| 2. | The reaction is endothermic |
| 3. | The product is more stable than substrate |
| 4. | The difference in average energy content of substrate from that of transition state is called activation energy. |
The role of an enzyme in a reaction is to/as
1.Decrease activation energy
2.Increase activation energy
3.Inorganic catalyst
4.Increase potential energy
Transition state structure of the substrate formed during an enzymatic reaction is
1.transient but stable
2.permanent but unstable
3.transient and unstable
4.permanent and stable







