The process of converting precipitate into collidal solution on adding an electrolyte is called:
(A) Peptisation
(B) Dialysis
(C) Electro-osmosis
(D) Eelectrophoresis
Emulsifying agent stabilized the emulsion of oil in water by
1. Forming an interfacial film between suspended particles and the medium
2. Dissolving the suspended particles into the medium completely
3. Coagulating the suspended particles from medium completely
4. Converting the suspended particles into vapor completely
If of charcoal having, active surface area 1000 is used for monolayer absorption of a gas having molecular radius cm, then volume of the absorbed gas at STP is [Avogadro's number = ]
1. 11.2 L
2. 22.4 L
3. 7.46 L
4. 3.73 L
Among the following, the surfactant that will form micelles in aqueous solution at the highest molar concentration
1.
2.
3.
4.
Some types of gels like gelatin liquify on shaking, thereby changing into sols. The sols on standing change back into a gel. The process is known as
1. Syneresis
2. Thixotropy
3. Peptisation
4. Imbition
A colloid of Agl is formed mixing with an excess of Kl. Which of the given electrolyte will have highest coagulation value for it?
1.
2. NaCl
3.
4.
The decreasing order of coagulating power of an electrolyte to coagulate the sol is
1.
2.
3.
4.
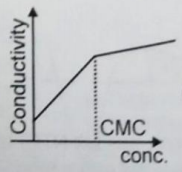
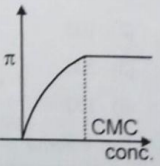
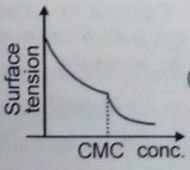
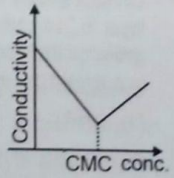
When detergent is added in water, some properties are measured with respect to concentration. Choose the incorrect plot.
1. 
2. 
3. 
4. 
The coagulation of 100 ml of colloidal solution of gold is completely prevented by the addition of 0.25 gm of a substance 'X' to it before adding 1 ml of 10% NaCl solution. The gold number of 'X' is
1. 25
2. 0.25
3. 2.5
4. 0.025
Which is correct in case of van der Waals adsorption?
1. High temperature; low pressure
2. Low temperature; high pressure
3. Low temperature; low pressure
4. High temperature; high pressure






