A gas such as carbon monoxide would be most likely to obey the ideal gas law at
(1) high temperatures and low pressures
(2) low temperatures and high pressures
(3) high temperatures and high pressures
(4) low temperatures and low pressures
A gaseous mixture was prepared by taking equal moles of CO and N2. If the total pressure of the mixture was found 1 atmosphere, the partial pressure of the nitrogen (N2) in the mixture is
(a) 0.8 atm
(b) 0.9 aim
(c) 1 atm
(d) 0.5 atm
What volume of oxygen gas (O2) measured at 0°C and 1 atm, is needed to burn completely 1 L of propane gas (C3H8) measured under the same conditions?
(1) 7L
(2) 6L
(3) 5L
(4) 10L
A certain volume of argon gas (Mol. wt. = 40) requires 45 s to effuse through a hole at a certain pressure and temperature. The same volume of another gas of unknown molecular weight requires 60s to pass through the same hole under the same conditions of temperature and pressure. The molecular weight of the gas is
(A) 53
(B) 35
(C) 71
(D) 120
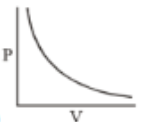
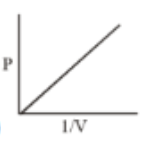
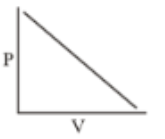
Which of the following curves does not represent Boyle's Law
(1) 
(2)
(3) 
(4) 
The ratio of average molecular kinetic energy of to that of , both at 300 K is
(A) 1 : 1
(B) 7 : 2
(C) 176 : 1
(D) 2 : 7
X ml of gas effuses through a hole in a container in 5 seconds. The time taken for the effusion of the same volume of the gas specified below under ideal condition is
(A) 10 seconds :
(B) 20 seconds :
(C) 25 seconds :
(D) 55 seconds :
The valves X and Y are opened simultaneously. The white fumes of will first form at :
(A) A
(B) B
(C) C
(D) A, B and C simultaneously
A gas mixture consists of 2 moles of oxygen and 4 moles of a argon at temperature T. Neglecting all vibrational modes, the total internal energy of the system is –
(A) 4RT
(B) 5RT
(C) 15RT
(D) 11RT
Two flasks of equal volume connected by a narrow tube (of negligible volume) at 27ºC and contain 0.70 mole of at 0.5 atm. One of the flasks is then immersed into a hot bath, kept at 127ºC, while the other remains at 27ºC. Calculate the final pressure.
(1) 5.714 atm
(2) 0.5714 atm
(3) 2.5214 atm
(4) 5.5114 atm








