Select Chapter Topics:
Given below are two statements:
| Assertion (A): | As a gas bubble rises from the bottom of a lake, its volume decreases. |
| Reason (R): | As the gas bubble rises from the bottom of a lake, the pressure of the gas within decreases. |
| 1. | (A) is True but (R) is False. |
| 2. | (A) is False but (R) is True. |
| 3. | Both (A) and (R) are True and (R) is the correct explanation of (A). |
| 4. | Both (A) and (R) are True but (R) is not the correct explanation of (A). |
Subtopic: Ideal Gas Equation |
68%
From NCERT
To view explanation, please take trial in the course.
NEET 2025 - Target Batch
Hints
To view explanation, please take trial in the course.
NEET 2025 - Target Batch
Given below are two statements:
| Assertion (A): | The translational kinetic energy of every molecule of an ideal gas increases by 50% if the absolute temperature is raised by \(50\text{%}.\) |
| Reason (R): | The average translational kinetic energy of the molecules of an ideal gas is directly proportional to its absolute temperature. |
| 1. | (A) is true but (R) is false. |
| 2. | (A) is false but (R) is true. |
| 3. | Both (A) and (R) are true and (R) is the correct explanation of (A). |
| 4. | Both (A) and (R) are true but (R) is not the correct explanation of (A). |
Subtopic: Kinetic Energy of an Ideal Gas |
From NCERT
To view explanation, please take trial in the course.
NEET 2025 - Target Batch
Hints
To view explanation, please take trial in the course.
NEET 2025 - Target Batch
Consider the following statements. The internal energy of an ideal monoatomic gas may have contributions from:
Which of the statements given above is/are correct?
| (1) | translational kinetic energy of its molecules |
| (2) | vibrational kinetic energy of its molecules |
| (3) | rotational kinetic energy of its molecules |
| (4) | potential energy corresponding to molecular forces |
Which of the statements given above is/are correct?
| 1. | (2) and (3) |
| 2. | (1) and (4) |
| 3. | (1) only |
| 4. | (1), (2), (3) and (4) |
Subtopic: Kinetic Energy of an Ideal Gas |
54%
From NCERT
To view explanation, please take trial in the course.
NEET 2025 - Target Batch
Hints
To view explanation, please take trial in the course.
NEET 2025 - Target Batch
The figure shows a plot of \(\frac{PV}{T}\) versus \(P\) for \(1.00 \times 10^{- 3}~\text{kg}\) of oxygen gas at two different temperatures.
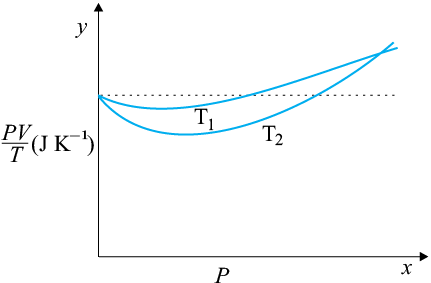
The value of \(\frac{PV}{T}\) where the curves meet on the \(y\)-axis is:
1. \(0.06~\text{JK}^{-1}\)
2. \(0.36~\text{JK}^{-1}\)
3. \(0.16~\text{JK}^{-1}\)
4. \(0.26~\text{JK}^{-1}\)
Subtopic: Ideal Gas Equation |
58%
To view explanation, please take trial in the course.
NEET 2025 - Target Batch
Hints
Links
To view explanation, please take trial in the course.
NEET 2025 - Target Batch
Given below are two statements:
| Assertion (A): | The molecules of a monoatomic gas has three degrees of freedom. |
| Reason (R): | The molecules of diatomic gas have five degrees of freedom. |
| 1. | Both (A) and (R) are True and (R) is the correct explanation of (A). |
| 2. | Both (A) and (R) are True but (R) is not the correct explanation of (A). |
| 3. | (A) is True but (R) is False. |
| 4. | Both (A) and (R) are False. |
Subtopic: Law of Equipartition of Energy |
92%
From NCERT
To view explanation, please take trial in the course.
NEET 2025 - Target Batch
Hints
To view explanation, please take trial in the course.
NEET 2025 - Target Batch
Given below are two statements:
| Assertion (A): | The total translational kinetic energy of all the molecules of a given mass of an ideal gas is 1.5 times the product of its pressure and volume. |
| Reason (R): | The molecules of gas collide with each other and the velocities of the molecules change due to the collision. |
| 1. | Both (A) and (R) are true and (R) is the correct explanation of (A). |
| 2. | Both (A) and (R) are true but (R) is not the correct explanation of (A). |
| 3. | (A) is true but (R) is false. |
| 4. | Both (A) and (R) are false. |
Subtopic: Kinetic Energy of an Ideal Gas |
66%
From NCERT
To view explanation, please take trial in the course.
NEET 2025 - Target Batch
Hints
To view explanation, please take trial in the course.
NEET 2025 - Target Batch
Given below are two statements:
| Assertion (A): | If a gas container in motion is suddenly stopped, the temperature of the gas rises. |
| Reason (R): | The kinetic energy of ordered mechanical motion is converted into the kinetic energy of random motion of gas molecules. |
| 1. | Both (A) and (R) are true and (R) is the correct explanation of (A). |
| 2. | Both (A) and (R) are true but (R) is not the correct explanation of (A). |
| 3. | (A) is true but (R) is false. |
| 4. | Both (A) and (R) are false. |
Subtopic: Kinetic Energy of an Ideal Gas |
62%
From NCERT
To view explanation, please take trial in the course.
NEET 2025 - Target Batch
Hints
To view explanation, please take trial in the course.
NEET 2025 - Target Batch
Given below are two statements:
| Assertion (A): | The internal energy of a real gas is a function of both, temperature and volume. |
| Reason (R): | Internal K.E. depends on temperature and internal P.E. depends on volume. |
| 1. | Both (A) and (R) are true and (R) is the correct explanation of (A). |
| 2. | Both (A) and (R) are true but (R) is not the correct explanation of (A). |
| 3. | (A) is true but (R) is false. |
| 4. | Both (A) and (R) are false. |
Subtopic: Kinetic Energy of an Ideal Gas |
50%
From NCERT
To view explanation, please take trial in the course.
NEET 2025 - Target Batch
Hints
To view explanation, please take trial in the course.
NEET 2025 - Target Batch
Given below are two statements:
| Assertion (A): | Absolute zero temperature is also the zero energy temperature of gas molecules. |
| Reason (R): | At absolute zero temperature, the molecules of gas come to rest, hence possess no energy of any form. |
| 1. | Both (A) and (R) are true and (R) is the correct explanation of (A). |
| 2. | Both (A) and (R) are true but (R) is not the correct explanation of (A). |
| 3. | (A) is true but (R) is false. |
| 4. | Both (A) and (R) are false. |
Subtopic: Kinetic Energy of an Ideal Gas |
From NCERT
To view explanation, please take trial in the course.
NEET 2025 - Target Batch
Hints
To view explanation, please take trial in the course.
NEET 2025 - Target Batch
Given below are two statements:
| Assertion (A): | The root mean square velocity of molecules of gas having a Maxwellian distribution of velocities is higher than their most probable velocity at any temperature. |
| Reason (R): | A very small number of molecules of a gas which possess very large velocities increase root mean square velocity, without affecting the most probable velocity. |
| 1. | Both (A) and (R) are true and (R) is the correct explanation of (A). |
| 2. | Both (A) and (R) are true but (R) is not the correct explanation of (A). |
| 3. | (A) is true but (R) is false. |
| 4. | Both (A) and (R) are false. |
Subtopic: Types of Velocities |
65%
From NCERT
To view explanation, please take trial in the course.
NEET 2025 - Target Batch
Hints
To view explanation, please take trial in the course.
NEET 2025 - Target Batch


