Which one of the following processes is reversible?
1.
Transfer of heat by radiation
2.
Transfer of heat by conduction
3.
Isothermal compression
4.
Electrical heating of a nichrome wire
An ideal gas heat engine operates in a Carnot cycle between 227ºC and 127ºC. It absorbs 6 × 104 cals of heat at higher temperatures.
The amount of heat converted to work will be?
1. 4.8 × 104 cals
2. 2.4 × 104 cals
3. 1.2 × 104 cals
4. 6 × 104 cals
If a gas changes volume from 2 litres to 10 litres at a constant temperature of 300K, then the change in its internal energy will be:
| 1. | 12 J | 2. | 24 J |
| 3. | 36 J | 4. | 0 J |
We consider a thermodynamic system. If ∆U represents the increase in its internal energy and W the work done by the system, which of the following statements is true?
| 1. | \(\Delta \mathrm{U}=-\mathrm{W}\) in an isothermal process |
| 2. | \(\Delta \mathrm{U}=\mathrm{W}\) in an isothermal process |
| 3. | \(\Delta \mathrm{U}=-\mathrm{W}\) in an adiabatic process |
| 4. | \(\Delta \mathrm{U}=\mathrm{W}\) in an adiabatic process |
At a pressure of \(2\) atmospheres, a mass of diatomic gas \((\gamma = 1.4)\), is compressed adiabatically, causing its temperature to rise from \(27^{\circ}\mathrm{C}\) to \(927^{\circ}\mathrm{C}\). The pressure of the gas in the final state is:
1. 8 atm
2. 28 atm
3. 68.7 atm
4. 256 atm
Consider a cycle followed by an engine (figure).
1 to 2 is isothermal,
2 to 3 is adiabatic,
3 to 1 is adiabatic.
Such a process does not exist, because:
| a. | heat is completely converted to mechanical energy in such a process, which is not possible. |
| b. | In this process, mechanical energy is completely converted to heat, which is not possible. |
| c. | curves representing two adiabatic processes don’t intersect. |
| d. | curves representing an adiabatic process and an isothermal process don't intersect. |
Choose the correct alternatives:
1. (a, b)
2. (a, c)
3. (b, c)
4. (c, d)
The figure shows the (P-V) diagram of an ideal gas undergoing a change of state from A to B. Four different paths I, II, III and IV, as shown in the figure, may lead to the same change of state.
| a. | The change in internal energy is the same in cases IV and III but not in cases I and II. |
| b. | The change in internal energy is the same in all four cases. |
| c. | Work done is maximum in case I. |
| d. | Work done is minimum in case II. |
Which of the following options contains only correct statements?
| 1. | (b), (c), (d) | 2. | (a), (d) |
| 3. | (b), (c) | 4. | (a), (c), (d) |
The pressure and volume of a gas are changed as shown in the P-V diagram. The temperature of the gas will:
| 1. | increase as it goes from A to B. |
| 2. | increase as it goes from B to C. |
| 3. | remain constant during these changes. |
| 4. | decrease as it goes from D to A. |
Two cylinders contain the same amount of an ideal monoatomic gas. The same amount of heat is given to two cylinders. If the temperature rise in cylinder A is T0, then the temperature rise in cylinder B will be:
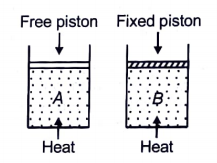
1.
2.
3.
4.
The incorrect relation is:
(where symbols have their usual meanings)
1.
2.
3.
4.





