The activation energy of a chemical reaction can be calculated by:
1. Determining the rate constant at standard temperature.
2. Determining the rate constant at two temperatures.
3. Determining probability of collision.
4. Using the catalyst.
The correct statement based on the graph below is:
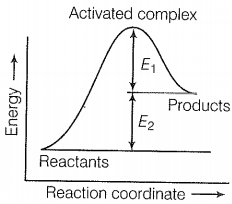
| 1. | The activation energy of the forward reaction is E1 + E2 and the product is less stable than reactant. |
| 2. | The activation energy of the forward reaction is E1 + E2 and the product is more stable than the reactant. |
| 3. | The activation energy of both forward and backward reaction is E1 + E2 and reactant is more stable than the product. |
| 4. | The activation energy of the backward reaction is E1 and the product is more stable than reactant. |
The correct graphical representation of relation between ln k and 1/T is:
| 1. |  |
2. |  |
| 3. |  |
4. |  |
Consider the Arrhenius equation given below and choose the correct option:
| 1. | Rate constant increases exponentially with increasing activation energy and decreasing temperature. |
| 2. | Rate constant decreases exponentially with increasing activation energy and increasing temperature. |
| 3. | Rate constant increases exponentially with decreasing activation energy and decreasing temperature. |
| 4. | Rate constant increases exponentially with decreasing activation energy and increasing temperature. |
The correct representation of an exothermic reaction is:
| 1. |  |
2. |  |
| 3. |  |
4. | Both 1 and 2 |
An incorrect statement about the collision theory of chemical reaction is:
| 1. | It considers reacting molecules or atoms to be hard spheres and ignores their structural features. |
| 2. | The number of effective collisions determines the rate of reaction. |
| 3. | The collision of atoms or molecules possessing sufficient threshold energy results in product formation. |
| 4. | Molecules should collide in the proper orientation for the collision to be effective with sufficient threshold energy and proper orientation. |
During decomposition of an activated complex
a. Energy is always released.
b. Energy is always absorbed.
c. Energy does not change.
d. Reactants may be formed.
The correct choice among the given is -
1. (a, b)
2. (b, c)
3. (c, d)
4. (a, d)
The correct statements among following about Maxwell, Boltzmann distribution of energy is -
| a. | The fraction of molecules with the most probable kinetic energy decreases at higher temperatures |
| b. | The fraction of molecules with the most probable kinetic energy increases at higher temperatures |
| c. | Most probable kinetic energy increases at higher temperatures |
| d. | Most probable kinetic energy decreases at higher temperatures |
1. (a, b)
2. (b, c)
3. (c, d)
4. (a, c)
The correct statements among the following regarding Maxwell, Boltzmann distribution curve of energy is -
| a. | The area under the curve must not change with an increase in temperature. |
| b. | The area under the curve increases with increase in temperature. |
| c. | Area under the curve decreases with increase in temperature. |
| d. | With an increase in temperature curve broadens and shifts to the right-hand side. |
1. (a, b)
2. (b, c)
3. (c, d)
4. (a, d)
The correct statements among the following regarding the Arrhenius equation is/are -
| a. | Rate of a reaction increases with an increase in temperature |
| b. | Rate of a reaction increases with a decrease in activation energy |
| c. | Rate constant decreases exponentially with an increase in temperature |
| d. | Rate of reaction decreases with a decrease in activation energy |
1. (a, b)
2. (b, c)
3. (c, d)
4. (a, d)


