The rate constant for the decomposition of hydrocarbons is 2.418 × 10–5 s–1 at 546 K. If the energy of activation is 179.9 kJ/mol,
the value of the pre-exponential factor will be:
For a reaction A → Product, with k = 2.0 × 10–2 s–1, if the initial concentration of A is 1.0 mol L-1, the concentration of A after 100 seconds would be :
| 1. | 0.23 mol L-1 | 2. | 0.18 mol L-1 |
| 3. | 0.11 mol L-1 | 4. | 0.13 mol L-1 |
The decomposition of sucrose follows the first-order rate law. For this decomposition, t1/2 is 3.00 hours. The fraction of a sample of sucrose that remains after 8 hours would be:
| 1. | 0.13 | 2. | 0.42 |
| 3. | 0.16 | 4. | 0.25 |
The decomposition of hydrocarbons follows the equation: k = (4.5 × 1011s–1) e-28000K/T
The activation energy (Ea) for the reaction would be:
| 1. | 232.79 kJ mol-1 | 2. | 245.86 kJ mol-1 |
| 3. | 126.12 kJ mol-1 | 4. | 242.51 kJ mol-1 |
The rate constant for the first-order decomposition of H2O2 is given by the equation: \(log \ k \ = \ 14.34 \ - \ 1.25 \ \times \ 10^{4}\frac{K}{T}\). The value of Ea for the reaction would be:
1. 249.34 kJ mol-1
2. 242.64 J mol-1
3. -275.68 kJ mol-1
4. 239.34 kJ mol-1
A first-order reaction takes 40 min for 30 % decomposition. The half life of the reaction will be:
| 1. | 88.8 min | 2. | 94.3 min |
| 3. | 67.2 min | 4. | 77.7 min |
The role of a catalyst is to change:
1. Gibbs energy of the reaction
2. Enthalpy of reaction
3. The activation energy of the reaction
4. Equilibrium constant
In the presence of a catalyst, the heat evolved or absorbed during the reaction:
1. Increases.
2. Decreases.
3. Remains unchanged.
4. May increase or decrease.
The activation energy of a chemical reaction can be calculated by:
1. Determining the rate constant at standard temperature.
2. Determining the rate constant at two temperatures.
3. Determining probability of collision.
4. Using the catalyst.
The correct statement based on the graph below is:
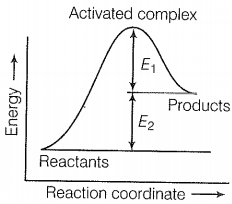
| 1. | The activation energy of the forward reaction is E1 + E2 and the product is less stable than reactant. |
| 2. | The activation energy of the forward reaction is E1 + E2 and the product is more stable than the reactant. |
| 3. | The activation energy of both forward and backward reaction is E1 + E2 and reactant is more stable than the product. |
| 4. | The activation energy of the backward reaction is E1 and the product is more stable than reactant. |


