The number of tetrahedral voids per unit cell in NaCl crystal is -
I. 4
II. 8
III. Twice the number of octahedral voids
IV. Four times the number of octahedral voids.
The correct choice among the given is-
1. (I, II)
2. (II, III)
3. (III, IV)
4. (I, IV)
Amorphous solids can also be called -
I. Pseudo solids
II. True solids
III. Supercooled liquids
IV. Supercooled solids
1. (I, II)
2. (II, III)
3. (III, IV)
4. (I, III)
A perfect crystal of silicon is doped with some elements as given in the options. Which of these options shows n-type semiconductors?
a. 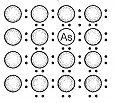 b.
b. 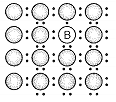
c. 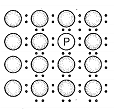 d.
d. 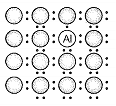
1. (a, b)
2. (b, c)
3. (c, d)
4. (a, c)
Which of the following statements is correct?
| I. | Ferrimagnetic substances lose ferrimagnetism on heating and become paramagnetic |
| II. | Ferrimagnetic substances do not lose ferrimagnetism on heating and remain ferrimagnetic |
| III. | Antiferromagnetic substances have domain structures similar to ferromagnetic substances and their magnetic moments are not canceled by each other |
| IV. | In ferromagnetic substances, all the domains get oriented in the direction of the magnetic field and remain as such even after removing the magnetic field |
The correct choice among the given is -
1. (I, II)
2. (II, III)
3. (III, IV)
4. (I, IV)
Match the defects given in Column I with the statements given in Column II.
| Column I | Column II |
| A. Simple vacancy defect | 1. Shown by non-ionic solids and increases the density of the solid |
| B. Simple interstitial defect | 2. Shown by ionic solids and decreases the density of the solid |
| C. Frenkel defect | 3. Shown by non-ionic solids and decreases the density of the solid |
| D. Schottky defect | 4. Shown by ionic solids and density of the solid remains the same |
Codes
| A | B | C | D | |
| 1. | 3 | 1 | 4 | 2 |
| 2. | 1 | 2 | 3 | 4 |
| 3. | 1 | 4 | 3 | 2 |
| 4. | 4 | 1 | 3 | 2 |
Match the type of unit cell given in Column I with the features given in Column II.
| Column I | Column II | |||
| A | Primitive cubic unit cell | 1. | Each of the three perpendicular edges compulsorily have the different edge length i.e., a ≠ b ≠ c | |
| B | Body centred cubic unit cell | 2. | Number of atoms per unit cell is one. | |
| C | Face centred cubic unit cell | 3. | Each of the three perpendicular edges compulsorily have the same edge length i.e., a=b=c | |
| D | End centred orthorhombic | 4. | In addition to the contribution from the corner unit cell atoms the number of atoms present in a unit cell is one | |
| 5. | In addition to the contribution from the corner atoms the number of atoms present in a unit cell is three | |||
Codes:
| Options: | A | B | C | D |
| 1. | 2,5 | 3,1 | 4,2 | 1,3 |
| 2. | 1 | 2 | 3 | 5 |
| 3. | 2,3 | 3,4 | 3,5 | 1,4 |
| 4. | 4 | 5 | 3 | 2 |
Match the types of defects given in Column I with the statement given in Column II.
| Column I | Column II |
| A. Impurity defect | 1. NaCl with anionic sites called F-centres |
| B. Metal excess defect | 2. FeO with Fe3+ |
| C. Metal deficiency defect | 3. NaCl with Sr2+ and some cationic sites vacant |
Codes
| A | B | C | |
| 1. | 2 | 3 | 1 |
| 2. | 3 | 1 | 2 |
| 3 | 1 | 2 | 3 |
| 4. | 2 | 1 | 3 |
Match the items given in Column I with the items given in Column II.
| Column I | Column II |
| A. Mg in solid state | 1. p-type semiconductor |
| B. MgCl2 in molten state | 2. n-type semiconductor |
| C. Silicon with phosphorus | 3. Electrolytic conductors |
| D. Germanium with boron | 4. Electronic conductors |
Codes
| A | B | C | D | |
| 1. | 2 | 3 | 4 | 1 |
| 2. | 1 | 2 | 3 | 4 |
| 3. | 1 | 4 | 3 | 2 |
| 4. | 4 | 3 | 2 | 1 |
Match the type of packing given in Column I with the items given in Column II.
| Column I | Column II |
| A. Square close packing in two dimensions | 1. Triangular voids |
| B. Hexagonal close packing in two dimensions | 2. Pattern of spheres is repeated in every fourth layer |
| C. Hexagonal close packing in three dimensions | 3. Coordination number = 4 |
| D. Cubic close packing in three dimensions | 4. Pattern of sphere is repeated in alternate layers |
Codes
| A | B | C | D | |
| 1. | 3 | 2 | 4 | 1 |
| 2. | 1 | 2 | 3 | 4 |
| 3. | 3 | 1 | 4 | 2 |
| 4. | 4 | 1 | 3 | 2 |
| Assertion (A): | The total number of atoms present in a simple cubic unit cell is one. |
| Reason (R): | Simple cubic unit cell has atoms at its corners, each of which is shared between eight adjacent unit cells. |
| 1. | Both (A) and (R) are true and (R) is the correct explanation of (A). |
| 2. | Both (A) and (R) are true but (R) is not the correct explanation of (A). |
| 3. | (A) is true but (R) is false. |
| 4. | (A) is false but (R) is true. |


