| 1. | decreases for conductors but increases for semiconductors. |
| 2. | increases for both conductors and semiconductors. |
| 3. | decreases for both conductors and semiconductors. |
| 4. | increases for conductors but decreases for semiconductors. |
The solids which have the negative temperature coefficient of resistance are:
| 1. | insulators only |
| 2. | semiconductors only |
| 3. | insulators and semiconductors |
| 4. | metals |
| 1. | in the case of \(\mathrm{C},\) the valence band is not completely filled at absolute zero temperature. |
| 2. | in the case of \(\mathrm{C},\) the conduction band is partly filled even at absolute zero temperature. |
| 3. | the four bonding electrons in the case of \(\mathrm{C}\) lie in the second orbit, whereas in the case of \(\mathrm{Si},\) they lie in the third. |
| 4. | the four bonding electrons in the case of \(\mathrm{C}\) lie in the third orbit, whereas for \(\mathrm{Si},\) they lie in the fourth orbit. |
1. \(6000~\mathring{A}\)
2. \(4000~\text{nm}\)
3. \(6000~\text{nm}\)
4. \(4000~\mathring{A}\)
A p-n photodiode is made of a material with a bandgap of 2.0 eV. The minimum frequency of the radiation that can be absorbed by the material is nearly:
1.
2.
3.
4.
In the energy band diagram of a material shown below, the open circles and filled circles denote holes and electrons respectively. The material is a/an:
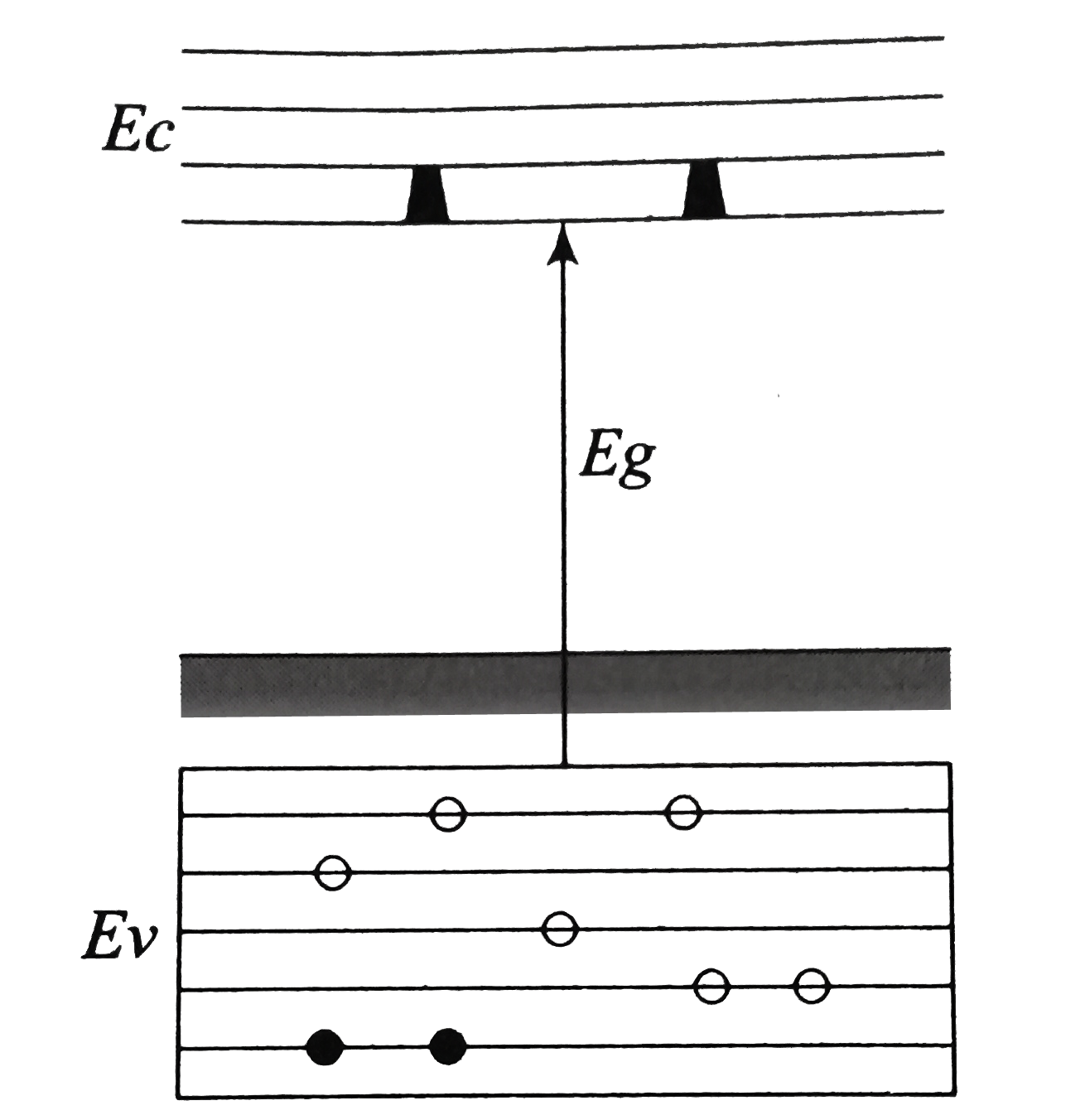
1. p-type semiconductor
2. insulator
3. metal
4. n-type semiconductor
| 1. | The resistivity of a semiconductor increases with an increase in temperature. |
| 2. | Substances with an energy gap of the order of \(10~\text{eV}\) are insulators. |
| 3. | In conductors, the valence and conduction bands may overlap. |
| 4. | The conductivity of a semiconductor increases with an increase in temperature. |
Carbon, Silicon, and Germanium atoms have four valence electrons each. Their valence and conduction bands are separated by energy band gaps represented by , and respectively. Which one of the following relationships is true in their case?
1.
2.
3.
4.
In semiconductors at room temperature:
| 1. | The valence band is completely filled and the conduction band is partially filled. |
| 2. | The valence band is completely filled. |
| 3. | The conduction band is completely empty. |
| 4. | The valence band is partially empty and the conduction band is partially filled. |






