The correct statement regarding polymeric silicon dioxide is -
| 1. | Each silicon atom is surrounded by four oxygen atoms and each oxygen atom is bonded to two silicon atoms. |
| 2. | Each silicon atom is surrounded by two oxygen atoms and each oxygen atom is bonded to two silicon atoms. |
| 3. | Silicon atom is bonded to two oxygen atoms. |
| 4. | These are double bonds between silicon and oxygen atoms. |
Subtopic: Properties of Structure of SiO2 & Other Compounds |
79%
Level 2: 60%+
Hints
Links
A compound among the following that is used in cosmetic surgery is -
| 1. | Silica | 2. | Silicates |
| 3. | Silicones | 4. | Zeolites |
Subtopic: Properties of Structure of SiO2 & Other Compounds |
74%
Level 2: 60%+
NEET - 2019
Hints
Links
Compound (A) is -
| 1. | A linear silicone | 2. | A chlorosilane |
| 3. | A linear silane | 4. | A network silane |
Subtopic: Properties of Structure of SiO2 & Other Compounds |
70%
Level 2: 60%+
Hints
Links
A compound among the following that is not a monomer for a high molecular mass silicone polymer is-
1.
2.
3.
4.
Subtopic: Properties of Structure of SiO2 & Other Compounds |
61%
Level 2: 60%+
Hints
Links
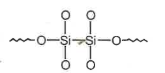
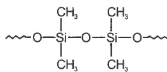
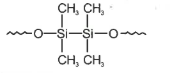
Hydrolysis of dimethyldichloro silane; \((Ch_3)_2SiCl_2\) followed by condensation polymerisation yields straight chain polymer of:
1.
2.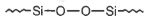
3.
4.
1.

2.
3.

4.

Subtopic: Properties of Structure of SiO2 & Other Compounds |
76%
Level 2: 60%+
Please attempt this question first.
Hints
Please attempt this question first.
When \(SiCl_4\) is allowed to undergo hydrolysis it gives:
1. \(SiO_2-\) Silica
2. \(Si(OH)_4-\) Silicic acid
3. \(Si(OH)Cl_3-\) Trichlorosilanol
4. \(SiCl_4\) do not undergo hydrolysis
1. \(SiO_2-\) Silica
2. \(Si(OH)_4-\) Silicic acid
3. \(Si(OH)Cl_3-\) Trichlorosilanol
4. \(SiCl_4\) do not undergo hydrolysis
Subtopic: Properties of Structure of SiO2 & Other Compounds |
82%
Level 1: 80%+
Please attempt this question first.
Hints
Given below are two statements:
| Assertion (A): | Silicones are water-repelling in nature. |
| Reason (R): | Silicones are organosilicon polymers, which have ( - R2SiO - ) as repeating units. |
| 1. | Both (A) and (R) are True and (R) is the correct explanation of (A). |
| 2. | Both (A) and (R) are True but (R) is not the correct explanation of (A). |
| 3. | (A) is True but (R) is False. |
| 4. | (A) is False but (R) is True. |
Subtopic: Properties of Structure of SiO2 & Other Compounds |
57%
Level 3: 35%-60%
Hints
Links
Unlock IMPORTANT QUESTION
This question was bookmarked by 5 NEET 2025 toppers during their NEETprep journey. Get Target Batch to see this question.
✨ Perfect for quick revision & accuracy boost
Buy Target Batch
Access all premium questions instantly
An anion present in the chain structure silicate is -
1.
2.
3.
4.
Subtopic: Properties of Structure of SiO2 & Other Compounds |
Level 3: 35%-60%
AIPMT - 2007
Hints
Links
\((\mathrm{Me})_2 \mathrm{SiCl}_2\) on hydrolysis will produce
1. \( (\mathrm{Me})_2 \mathrm{Si}(\mathrm{OH})_2 \)
2. \((\mathrm{Me})_2 \mathrm{Si}=\mathrm{O} \)
3. \( -\left[-\mathrm{O}-(\mathrm{Me})_2 \mathrm{Si}-\mathrm{O}-\right]_{\mathrm{n}}- \)
4. \(\mathrm{Me}_2 \mathrm{SiCl}(\mathrm{OH})\)
1. \( (\mathrm{Me})_2 \mathrm{Si}(\mathrm{OH})_2 \)
2. \((\mathrm{Me})_2 \mathrm{Si}=\mathrm{O} \)
3. \( -\left[-\mathrm{O}-(\mathrm{Me})_2 \mathrm{Si}-\mathrm{O}-\right]_{\mathrm{n}}- \)
4. \(\mathrm{Me}_2 \mathrm{SiCl}(\mathrm{OH})\)
Subtopic: Properties of Structure of SiO2 & Other Compounds |
60%
Level 2: 60%+
Please attempt this question first.
Hints
Please attempt this question first.
Given below are two statements:
| Assertion (A): | If aluminium atoms replace a few silicon atoms in three-dimensional network of silicon dioxide, the overall structure acquires a negative charge. |
| Reason (R): | Aluminium is trivalent while silicon is tetravalent. |
| 1. | Both (A) and (R) are True and (R) is the correct explanation of (A). |
| 2. | Both (A) and (R) are True but (R) is not the correct explanation of (A). |
| 3. | (A) is True but (R) is False. |
| 4. | (A) is False but (R) is True. |
Subtopic: Properties of Structure of SiO2 & Other Compounds |
64%
Level 2: 60%+
Hints






