An ideal gas goes from A to B via two processes, l and ll, as shown. If and are the changes in internal energies in processes I and II, respectively, then (\(P:\) pressure, \(V:\) volume)
| 1. | ∆U1 > ∆U2 | 2. | ∆U1 < ∆U2 |
| 3. | ∆U1 = ∆U2 | 4. | ∆U1 ≤ ∆U2 |
Prefer Books for Question Practice? Get NEETprep's Unique MCQ Books with Online Audio/Video/Text Solutions via Telegram Bot
NEET MCQ Books for XIth & XIIth Physics, Chemistry & Biology(where symbols have their usual meanings)
1. \(C_P = \frac{\gamma R}{\gamma-1 }\)
2. \(C_P-C_V= R\)
3. \(\Delta U = \frac{P_fV_f-P_iV_i}{1-\gamma}\)
4. \(C_V = \frac{R}{\gamma-1 }\)
Prefer Books for Question Practice? Get NEETprep's Unique MCQ Books with Online Audio/Video/Text Solutions via Telegram Bot
NEET MCQ Books for XIth & XIIth Physics, Chemistry & BiologyIf n moles of an ideal gas is heated at a constant pressure from 50°C to 100°C, the increase in the internal energy of the gas will be: \(\left(\frac{C_{p}}{C_{v}} = \gamma\ and\ R = gas\ constant\right)\)
| 1. | \(\frac{50 nR}{\gamma - 1}\) | 2. | \(\frac{100 nR}{\gamma - 1}\) |
| 3. | \(\frac{50 nγR}{\gamma - 1}\) | 4. | \(\frac{25 nγR}{\gamma - 1}\) |
Prefer Books for Question Practice? Get NEETprep's Unique MCQ Books with Online Audio/Video/Text Solutions via Telegram Bot
NEET MCQ Books for XIth & XIIth Physics, Chemistry & BiologyWhen an ideal diatomic gas is heated at constant pressure, the fraction of the heat energy supplied which increases the internal energy of the gas is?
| 1. | \(2 \over 5\) | 2. | \(3 \over 5\) |
| 3. | \(3 \over 7\) | 4. | \(5 \over 7\) |
Prefer Books for Question Practice? Get NEETprep's Unique MCQ Books with Online Audio/Video/Text Solutions via Telegram Bot
NEET MCQ Books for XIth & XIIth Physics, Chemistry & BiologyTwo cylinders contain the same amount of an ideal monoatomic gas. The same amount of heat is given to two cylinders. If the temperature rise in cylinder A is T0, then the temperature rise in cylinder B will be:
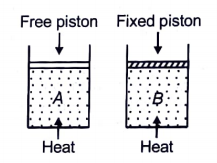
1.
2.
3.
4.
Prefer Books for Question Practice? Get NEETprep's Unique MCQ Books with Online Audio/Video/Text Solutions via Telegram Bot
NEET MCQ Books for XIth & XIIth Physics, Chemistry & BiologyThe specific heat of a gas in an isothermal process is:
| 1. | Infinite | 2. | Zero |
| 3. | Negative | 4. | Remains constant |
Prefer Books for Question Practice? Get NEETprep's Unique MCQ Books with Online Audio/Video/Text Solutions via Telegram Bot
NEET MCQ Books for XIth & XIIth Physics, Chemistry & BiologyThe volume (\(V\)) of a monatomic gas varies with its temperature (\(T\)), as shown in the graph. The ratio of work done by the gas to the heat absorbed by it when it undergoes a change from state \(\mathrm{A}\) to state \(\mathrm{B}\) will be:
| 1. | \(2 \over 5\) | 2. | \(2 \over 3\) |
| 3. | \(1 \over 3\) | 4. | \(2 \over 7\) |
Prefer Books for Question Practice? Get NEETprep's Unique MCQ Books with Online Audio/Video/Text Solutions via Telegram Bot
NEET MCQ Books for XIth & XIIth Physics, Chemistry & Biology

