The correct statement is-
1. When O2 is converted into then bond distance increases
2. When N2 is converted into then bond distance increases
3. When CO is converted into CO+ then bond distance increases
4. All of the above

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
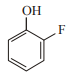
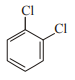
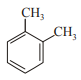
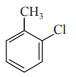
In which case observed dipole moment is greater than theoretical dipole moment?
1. 
2. 
3. 
4. 
Which has linear shape but not sp hybridisation?
1. XeF2
2.
3. CO2
4. Both 1 and 2

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Which one of the following pairs is iso-structural?
(i.e., having the same shape and hybridization)
1.
2.
3.
4.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
In which of the following molecules are all the bonds not equal?
1.
2.
3.
4.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
The favourable conditions for the formation of electrovalency is
1. Small cation and small anion
2. Large cation and small anion
3. Small cation and large anion
4. Large cation and large anion

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.



