How will you distinguish 1° and 2° hydroxyl groups present in glucose? Explain with reactions.
1° and 2° hydroxyl groups present in glucose can be identified by the reaction of glucose with nitric acid. THE primary OH group present in glucose is easily oxidized to-COOH group while the secondary OH group does not.
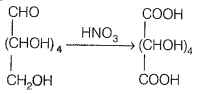
Hence, one OH is the primary OH group

© 2026 GoodEd Technologies Pvt. Ltd.