Which one of the following reactions of glucose can be explained only by its cyclic structure?
| 1. | Glucose forms pentaacetate. |
| 2. | Glucose reacts with hydroxylamine to form an oxime. |
| 3. | Pentaacetate of glucose does not react with hydroxylamine. |
| 4. | Glucose is oxidized by nitric acid to saccharic acid. |
HINT: Penta-acetate of glucose does not react with hydroxylamine.
Explanation:
1. Glucose reacts with acetic anhydride to form penta-acetate. It confirms the presence of five -OH groups in glucose which are attached to different carbon atoms.
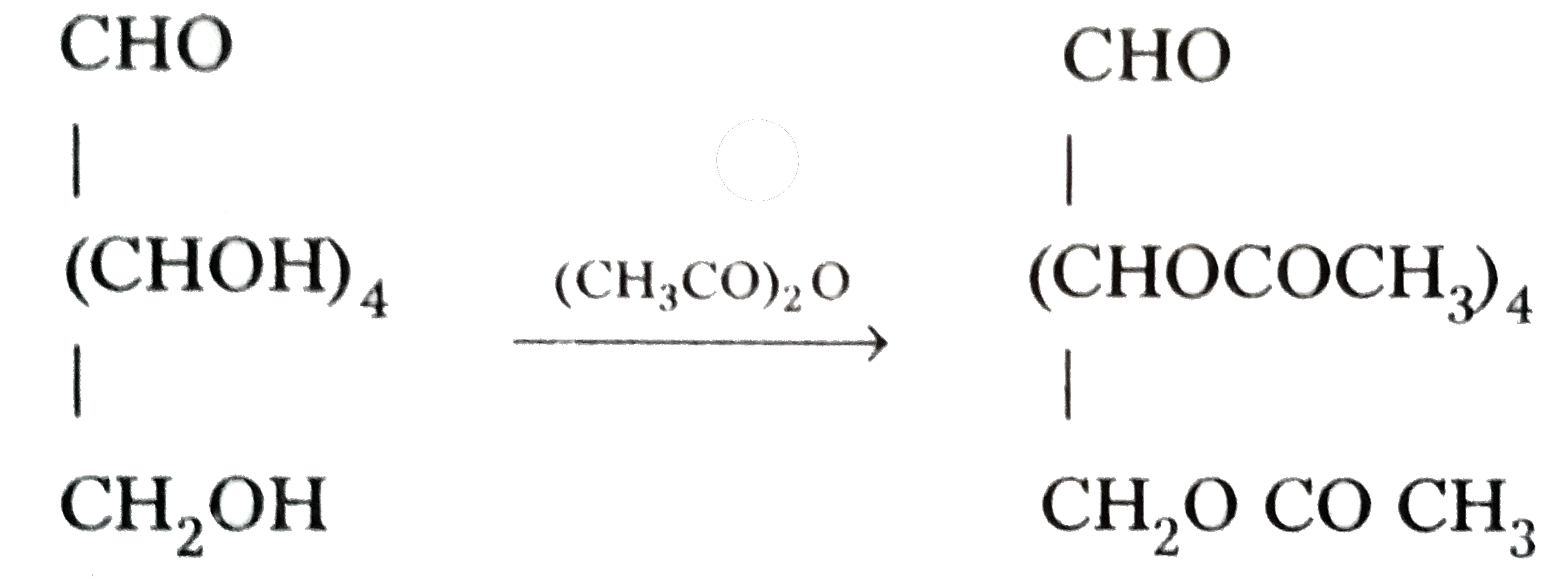
2. Glucose reacts with hydroxylamine to form an oxime.
3. "Pentaacetate of glucose does not react with hydroxylamine" showing the absence of free CHO group.
This can not be explained by the open structure of glucose. while all other properties are easily explained by open structure or glucose.
4. On being treated with HNO3, D-glucose get oxidized to give saccharic acid.
Hence, the option (3) is the correct choice.

© 2026 GoodEd Technologies Pvt. Ltd.

