Synthetic detergents have an advantage over usual soaps as far as cleansing power is concerned. But the use of synthetic detergents over a long time creates environmental pollution. How can the pollution caused by synthetic detergents be minimized? Classify the detergents according to their chemical nature.
Synthetic detergents are cleansing agents that have all the properties of soaps, but which actually do not contain any soap. These can be used in soft as well as in hard water.
They are mainly classified into three categories
(1) Anionic Detergents
Anionic detergents are sodium salts of sulphonated long-chain alcohols or hydrocarbons. Alkyl hydrogen sulfates formed by treating long-chain alcohols with conc. H, SO, is neutralized with alkali to form anionic detergents. Similarly, alkyl benzene sulphonates are obtained by neutralizing alkyl benzene sulphonic acids with alkali.
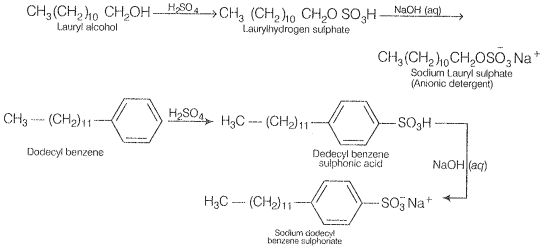
In these detergents, the anionic part of the molecule is involved in the cleansing action. They are mostly used for household work. They are also used in toothpaste.
(2) Cationic Detergents
They are quarternary ammonium salts of amines with acetates, chlorides, or bromides as anions. The cationic part possesses a long hydrocarbon chain and a positive charge on the nitrogen atom. Cetyltrimethylammonium bromide is a popular cationic detergent and is used in hair conditioners.
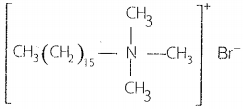
Cationic detergents have germicidal properties and are expensive, therefore, these are of limited use.
(3) Non-ionic Detergents
Non-ionic detergents do not contain any ion in their constitution. One such detergent is formed when stearic acid reacts with polyethylene glycol.
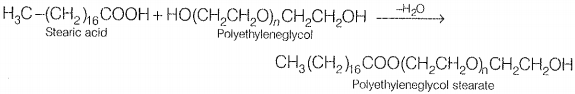
Liquid dishwashing detergents are non-ionic type Advantages of Synthetic Detergents over Soaps
(i) Synthetic detergents can be used in hard water without any wastage while some of the soaps get wasted.
(ii) Synthetic detergents can be used in acidic medium while soaps get precipitated.
(iii) Synthetic detergents are more soluble in water and hence produce father more easily than soaps. Some synthetic detergents produce lather even in ice-cold water.
(iv) Synthetic detergents decrease the surface tension of water to a greater extent and hence have a stronger cleansing action than soap.
Synthetic detergents have advantages over usual soaps but the use of synthetic detergents over a long time creates environmental pollution because some detergents have highly branched hydrocarbon chains.
These branches or side chains stop bacteria from attacking and breaking the chains. This results in the slow degradation of the detergent molecule leading to their accumulation. Effluents containing these detergents reach the rivers, ponds, etc. These persist in water even after sewage treatment and thus water gets polluted
Since, unbranched (i.e., straight) chains are more prone to attack by bacteria, therefore, in most of the detergents used these days, the branching is kept to a minimum so that the detergent becomes easily biodegradable and hence pollution is prevented.