1. CH3NH2
2. NCCH2NH2
3. (CH3)2NH
4. C6H5NHCH3
| Compounds | Factors responsible for basic character are |
| CH3-NH2 | Inductive effect (+ I) |
| NC-CH2-NH2 | Inductive effect (- I) |
| (CH3)2NH | Inductive effect (+ I) and Solvation |
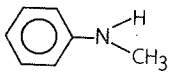 |
- I effect and resonance |

© 2026 GoodEd Technologies Pvt. Ltd.