An alkene ‘A’ (molecular formula ) on ozonolysis gives a mixture of two compounds ’B’ and ’C. Compound ‘8’ gives positive Fehling’s test and also forms iodoform on treatment with l, and NaOH. Compound ‘C’ does not give Fehling’s test but forms iodoform. Identify the compounds A, B and C. Write the reaction for ozonolysis and formation of iodoform from B and C.
Degree of unsaturation =
where C = number of carbon atoms
H = number of hydrogen atoms
Compound A will be either alkene or cyclic hydrocarbon. Since, A is undergoing ozonolysis hence A must be an alkene.
Possible structures of alkene are
l.
ll.
lll..
lV.
Ozonolysis of structure I produces aldehyde only
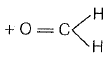
Ozonolysis of structure II produces aldehyde only
Ozonolysis of structure III Produces aldehyde only
Ozonolysis of structure IV produces both aldehyde and ketone
After ozonolysis of each of structures I, II and III produces only aldehydes as both Components. But as given in the question one compound doesnt give Fehling test but must give iodoform test. Hence, compound must be a ketone with
group. Hence, correct structure is IV.
Formation of iodoform from ‘B’ and ‘C’ may be explained as follows

© 2026 GoodEd Technologies Pvt. Ltd.