The correct order of increasing acidic strength is:
| 1. | Phenol < Ethanol < Chloroacetic acid < Acetic acid |
| 2. | Ethanol < Phenol < Chloroacetic acid < Acetic acid |
| 3. | Ethanol < Phenol < Acetic acid < Chloroacetic acid |
| 4. | Chloroacetic acid < Acetic acid < Phenol < Ethanol |
Hint: Acids are more acidic than phenol
Phenol is more stable than alcohol due to formation of more stable conjugate base (resonance stabilized) after removal of from phenol.
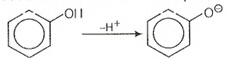
On the other hand, carboxylic acid is more acidic than phenol due to formation of more stable conjugate base after removal of as compared to phenol.
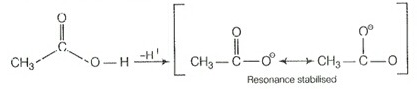
Chloroacetic acid is more acidic than acetic acid due to the presence of electron withdrawing chlorine group attached to carbon of carboxylic acid.
The overall order is as follows:
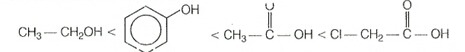
Hence, correct choice is (3).

© 2026 GoodEd Technologies Pvt. Ltd.