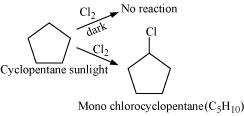
10.5 A hydrocarbon C5H10 does not react with chlorine in dark but gives a single monochloro compound C5H9Cl in bright sunlight. Identify the hydrocarbon.
A hydrocarbon with the molecular formula, (CH) belongs to the group with a general
molecular formula CH. Therefore, it may either be an alkene or a cycloalkane. Since
hydrocarbon does not react with chlorine in the dark, it cannot be an alkene. Thus, it
should be a cycloalkane.
Further, the hydrocarbon gives a single monochloro compound, CHCl by reacting with
chlorine in bright sunlight. Since a single monochloro compound is formed, the
hydrocarbon must contain H−atoms that are all equivalent. Also, as all H−atoms of a
cycloalkane are equivalent, the hydrocarbon must be a cycloalkane. Hence, the said
compound is cyclopentane.
Cyclopentane (CH)
The reactions involved in the question are:

© 2026 GoodEd Technologies Pvt. Ltd.