Question 13.10:-
Why is benzene extraordinarily stable though it contains three double bonds?
Benzene is a hybrid of resonating structures given as:
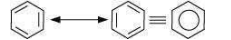
All six carbon atoms in benzene are hybridized. The two hybrid orbitals of each carbon atom overlap with the hybrid orbitals of adjacent carbon atoms to form six sigma bonds in the hexagonal plane. The remaining hybrid orbital on each carbon atom overlaps with the s-orbital of hydrogen to form six sigma C–H bonds. The remaining unhybridized p-orbital of carbon atoms has the possibility of forming three π bonds by the lateral overlap of
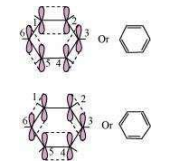
The six π’s are delocalized and can move freely about the six-carbon nuclei. Even after the presence of three double bonds, these delocalized π-electrons stabilize benzene.

© 2026 GoodEd Technologies Pvt. Ltd.