Question 13.9:-
Draw the cis and trans structures of hex-2-ene. Which isomer will have higher b.p. and why?
Hex-2-ene is represented as:
Geometrical isomers of hex-2-ene are:
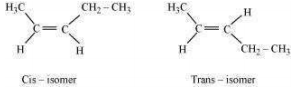
The dipole moment of cis-compound is a sum of the dipole moments of bonds acting in the same direction.
The dipole moment of trans-compound is the resultant of the dipole moments of bonds acting in opposite directions.
Hence, cis-isomer is more polar than trans-isomer. The higher the polarity, the greater is the intermolecular dipole-dipole interaction and the higher will be the boiling point.
Hence, cis-isomer will have a higher boiling point than trans-isomer.

© 2026 GoodEd Technologies Pvt. Ltd.