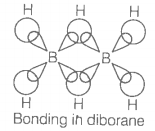
In the structure of diborane,
| 1. | All hydrogen atoms lie in one plane and boron atoms lie in a plane perpendicular to this plane. |
| 2. | 2 boron atoms and 4 terminal hydrogen atoms lie in the same plane and 2 bridging hydrogen atoms lie in the perpendicular plane. |
| 3. | 4 bridging hydrogen atoms and boron atoms lie in one plane and two terminal hydrogen atoms lie in a plane perpendicular to this plane. |
| 4. | All the atoms are in the same plane. |
 and
and 

© 2026 GoodEd Technologies Pvt. Ltd.