9.33 What do you expect the nature of hydrides is, if formed by elements of atomic numbers 15, 19, 23 and 44 with dihydrogen? Compare their behaviour towards water.
1) Hydride of nitrogen
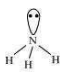
Hydride of nitrogen () is a covalent molecule. It is an electron-rich hydride owing to the presence of excess electrons as a lone pair on nitrogen.
2) Hydride of potassium
Dihydrogen forms an ionic hydride with potassium owing to the high electropositive nature of potassium. It is crystalline and non-volatile in nature.
3) Hydrides of Vanadium and Ruthenium
Both vanadium and ruthenium belong to the d–block of the periodic table. The metals of d–block form metallic or non–stoichiometric hydrides. Hydrides of vanadium and ruthenium are therefore, metallic in nature having a deficiency of hydrogen.
4) Behaviour of hydrides towards water
Potassium hydride reacts violently with water as:
Ammonia () behaves as a Lewis base and reacts with water as:
Hydrides of vanadium and Ruthenium do not react with water. Hence, the increasing order of reactivity of the hydrides is (V, Ru) H < < KH.

© 2026 GoodEd Technologies Pvt. Ltd.