For a hydride to act as a Lewis acid i.e., electron accepting, it should be electrondeficient. Also, for it to act as a Lewis base i.e., electron donating, it should be electronrich. Taking as an example, the total number of electrons are 14 and the total covalent bonds are seven. Hence, the bonds are regular 2e– -2 centered bonds.
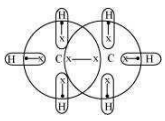
Hence, hydride has sufficient electrons to be represented by a conventional Lewis structure. Therefore, it is an electron-precise hydride, having all atoms with complete octets. Thus, it can neither donate nor accept electrons to act as a Lewis acid or Lewis base.

© 2026 GoodEd Technologies Pvt. Ltd.