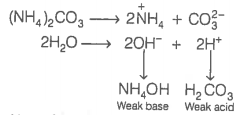
Given below are two statements:
| Assertion (A): | An aqueous solution of ammonium carbonate is basic. |
| Reason (R): |
The acidic or basic nature of a salt solution of a salt of a weak acid and a weak base depends on the Ka and Kb values of the acid and the base forming it. |
| 1. | Both (A) and (R) are True and (R) is the correct explanation of (A). |
| 2. | Both (A) and (R) are True but (R) is not the correct explanation of (A). |
| 3. | (A) is True but (R) is False. |
| 4. | (A) is False but (R) is True. |

© 2026 GoodEd Technologies Pvt. Ltd.