5.21 In terms of Charles’ law explain why –273 °C is the lowest possible temperature.
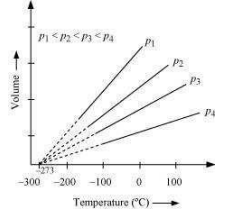
It was found that for all gases (at any given pressure), the plots of volume vs. temperature (in °C) is a straight line. If this line is extended to zero volume, then it intersects the temperature-axis at – 273°C. In other words, the volume of any gas at – 273°C is zero.
This is because all gases get liquefied before reaching a temperature of – 273°C. Hence, it can be concluded that – 273°C is the lowest possible temperature.

© 2026 GoodEd Technologies Pvt. Ltd.