The molecule among the following having the highest dipole moment is:
1. CO2
2. HI
3. H2O
4. SO2
HINT: If the molecule is symmetrical then its dipole moment is zero.
Explanation:
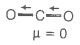
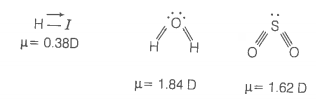

© 2026 GoodEd Technologies Pvt. Ltd.