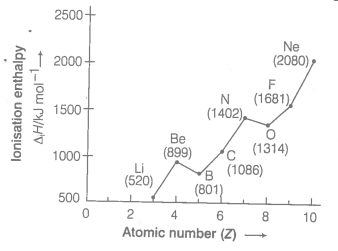
Ionisation enthalpies of elements of second period are given below
Ionisation enthalpy/k cal mol-1: 520, 899, 801, 1086, 1402, 1314, 1681, 2080.
Match the correct enthalpy with the elements and complete the graph given in figure. Also write symbols of elements with their atomic number.

© 2026 GoodEd Technologies Pvt. Ltd.