Hint: The total no. of molecules will be equal to the difference of molecules going outside and coming inside.
Step 1: Find the no. of molecules colliding the wall in time interval t.
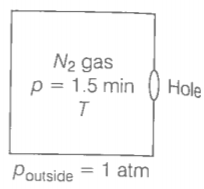
Given, the volume of the box, V=1.00
Temperature outside = Temperature inside
Initial pressure inside the box = 1.50 atm.
Final pressure inside the box = 0.10 atm.
Assuming,
= Speed of nitrogen molecule inside the box along the x-direction.
= number of molecules per unit volume colliding the wall in a time interval of t
All the particles at a distance will collide with the hole and the wall, the particle colliding along the hole will escape out reducing the pressure in the box.
Let area of the wall = A
The number of particles colliding in time A
is the factor because all the particles along x-direction are behaving randomly. Hence, half of these are colliding against the walls on either side.
Inside the box,
or
[According to the kinetic theory of gases]
Now,
Or
Number of particles colliding in time
Step 2: Find the difference of molecules going outside and coming inside.
If particles collide along the hole, they move out. Similarly, outside particles colliding along the hole will move inside.
If a = area of hole
Then, net particle flow in time
[Temperatures inside and outside the box are equal]
Let n= number density of nitrogen
Let = Avogardro's number
If after time t, the pressure inside changes from p to ;
Step 3: Find the after which the pressure reduces to the final pressure.
Now, number of molecules gone out
Putting the values from the data given,