When an ideal monoatomic gas is heated at constant pressure, fraction of heat energy supplied which increases the internal energy of gas, is
(1) 2/5
(2) 3/5
(3) 3/7
(4) 3/4
A container of volume 1 is divided into two equal compartments by a partition. One of these compartments contains an ideal gas at 300 K. The other compartment is the vacuum. The whole system is thermally isolated from its surroundings. The partition is removed and the gas expands to occupy the whole volume of the container. Its temperature now would be
1. 300 K
2. 239 K
3. 200 K
4. 100 K
A perfect gas goes from state A to another state B by absorbing 8x Jof heat and doing 6.5x J of external work. It is now transferred between the same two states in another process in which it absorbs J of heat. Then in the second process
1. Work done on the gas is 0.5x J
2. Work done by the gas is 0.5x J
3. Work done on the gas is J
4. Work done by the gas is J
The specific heat of hydrogen gas at constant pressure is = 3.4x cal/kg °C and at constant volume is = 2.4x cal/kg °C. If one-kilogram hydrogen gas is heated from 10°C to 20°C at constant pressure, the external work done on the gas to maintain it at constant pressure is
1. cal
2. cal
3. cal
4. 5x cal
One mole of an ideal gas expands at a constant temperature of \(300~\text{K}\) from an initial volume of \(10\) litres to a final volume of \(20\) litres.
The work done in expanding the gas is equal to:
(\(R = 8.31\) J/mole-K)
1. \(750~\text{J}\)
2. \(1728~\text{J}\)
3. \(1500~\text{J}\)
4. \(3456~\text{J}\)
An ideal gas at 27°C has compressed adiabatically to 8/27 of its original volume. If = 5/3, then the rise in temperature is
1. 450 K
2. 375 K
3. 225 K
4. 405 K
The pressure and density of a diatomic gas ( =7/5) change adiabatically from (P,d) to (P”,d”). If = 32, then should be
1. 1/128
2. 32
3. 128
4. None of the above
A thermally insulated vessel contains an ideal gas of molecular mass m and the ratio of specific heats . It is moving with speed v and is suddenly brought to rest. Assuming no heat is lost to the surroundings, its temperature increases
1.
2.
3.
4.
Consider a spherical shell of radius \(R\) at temperature \(T.\) The black body radiation inside it can be considered as an ideal gas of photons with internal energy per unit volume \(u=\dfrac{U}{v}\propto T^4\) and pressure\(P=\dfrac{1}{3}\dfrac{U}{v}.\) If the shell now undergoes an adiabatic expansion the relation between \(T\) and \(R\) is
1.
2.
3.
4.
A solid of constant heat capacity 1 J/°C is being heated by keeping it in contact with reservoirs in two ways
(i) Sequentially keeping in contact with 2 reservoirs such that each reservoir supplies the same amount of heat
(i) Sequentially keeping in contact with 8 reservoirs supplies the same amount of heat In both the case's body is brought from initial temperature 100°C to final temperature 200°C. Entropy change of the body in the two cases respectively is
1. In2, 41n2
2. In2, In2
3. In2, 21n2
4. 21n2, 8in2
In a cornot engine, when T2 =00 C and T1 =2000 C, its efficiency is \(\eta _{1}\) and when T1 =00 C and T2 =-2000 C. Its efficiency is \(\eta _{2}\), then what is \(\frac{\eta _{1}}{\eta 2}?\)
1. 0.577
2. 0.733
3. 0.638
4. Can't be calculated
A refrigerator with coefficient of performance 1/3 releases 200 J of heat to a hot reservoir. Then the work done on the working substance is
1. 100 J
2. 200/3 J
3. 50 J
4. 150 J
A refrigerator works between and . It is required to remove 600 calories of heat every second in order to keep the temperature of the refrigerated space constant. The power is
1. 2.365 W
2. 23.65 W
3. 236.5 W
4. 2365 W
1 cm3 of water at its boiling point absorbs 540 calories of heat to become steam with a volume of 1671 cm3. If the atmospheric pressure= and the mechanical equivalent of heat=4.19 J/calorie, the energy spent in this process in overcoming intermolecular forces is
1. 540 cal
2. 40 cal
3. 500 cal
4. Zero
One mole of a diatomic ideal gas undergoes a cyclic process ABC as shown in figure. The process BC is adiabatic. The temperatures at A, B and C are 400 K, 800 K and 600 K respectively. Choose the correct statement 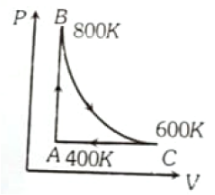
1. The change in internal energy in whole cyclic process is 250 R
2. The change in internal energy in the process CA is 700 R
3. The change in internal energy in the process AB is -350 R
4. The change in internal energy in the process BC is -500 R
A monoatomic ideal gas, intially at temperature T, is enclosed in a cylinder fitted with frictionless piston. The gas is allowed to expand adiabatically to a temperature T by releasing the piston suddenly. if Lamd L are the lengths of the gas column before and after expansion respectively, then T/T is given by
1.
2.
3.
4.
Two moles of ideal helium gas are in a rubber balloon at . The balloon is fully expandable and can be assumed to require no energy in its expansion. The temperature of the gas in the balloon is slowly changed to . The amount of heat required in raising the temperature is nearly (take R=8.31 J/mol K)
1. 62 J
2. 104 J
3. 124 J
4. 208 J
An ideal gas is subjected to cyclic process involving four thermodynamic states, the amounts of heat (Q) and Work (W) involved in each of these
The ratio of the net work done by the gas to the total heat absorbed by the gas is . The values of x and respectively are
1. 500; 7.5%
2. 700; 10.5%
3. 1000; 21%
4. 1500; 15%
Two cylinders A and B fitted with pistons contain equal amounts of an ideal diatomic gas at 300 K. The piston of A is free to move while that of B is held fixed. The same amount of heat is given to the gas in each cylinder. If the rise in temperature of the gas in A is 30 K, then the rise in temperature of the gas in B is
1. 30 K
2. 18 K
3. 50 K
4. 42 K
Initial pressure and volume of a gas are P and V respectively. First it is expanded isothermally to volume 4V and then compressed adiabatically to volume V. The final pressure of gas will be
1. 1P
2. 2P
3. 4P
4. 8P
A system goes from A to B via two processes I and II as shown in figure. If are the changes in internal energies in the processes I and II respectively, then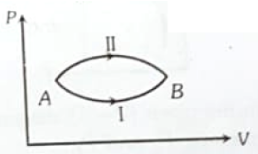
1.
2.
3.
4. Relation between cannot be determined
A thermodynamic system is taken through the cycle PQRSP process. The net work done by the system is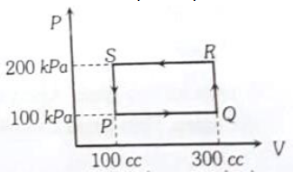
1. 20 J
2. -20 J
3. 400 J
4. -374 J
An ideal gas goes from state A or B via three different processes as indicated by P-V diagram. If indicate the gas absorbed by the gas along the three processes and indicate the change in internal energy along the three processes respectively, then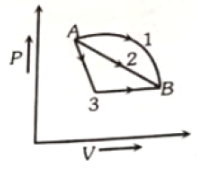
1.
2.
3.
4.
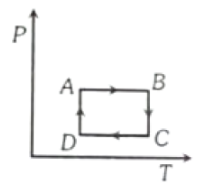
A cyclic process ABCD is shown in figure P-V diagram. Which of the following curves represent the same process?
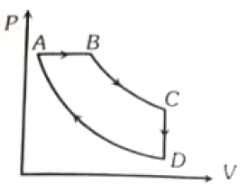
1. 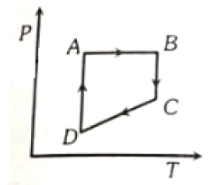 2.
2. 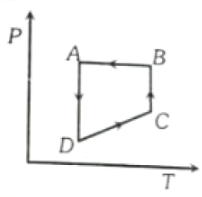
3. 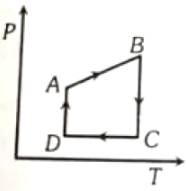 4.
4. 
The temperature-entropy diagram of a reversible engine cycle is given in the figure. Its efficiency is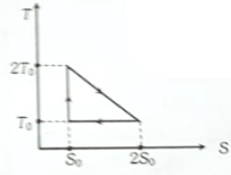
1. 1/3
2. 2/3
3. 1/2
4. 1/4
One mole of an ideal gas having initial volume V, pressure 2P and temperature T undergoes a cyclic process ABCDA as shown in below. The work done by the gas in the complete cycle is: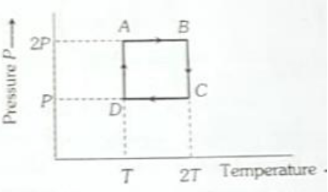
1. Zero
2.
3.
4.
The P-V graph of an ideal grass cycle is shown here as below. The adiabatic process is described by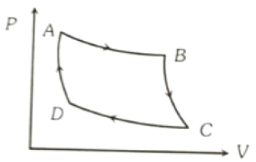
1. AB and BC
2. AB and CD
3. BC and DA
4. BC and CD
A thermodynamic system undergoes cyclic process ABCDA as shown in figure. The work done by the system is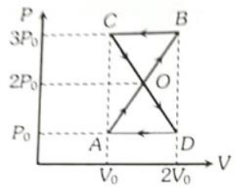
1.
2.
3.
4. Zero
The above p-v diagram represents the thermodynamic cycle of an engine, operating with an ideal monoatomic gas. The amount of heat, extracted from the source in a single cycle is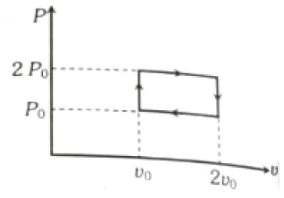
1.
2.
3.
4.