Which gas will be adsorbed on a solid to greater extent?
1. Having non-polar molecule
2. Having highest critical temperature
3. Having lowest critical temperature
4. Having lowest critical pressure
The volume of gases , and adsorbed by one gram of charcoal at 300 K are in order of :
1. > >
2. > >
3. > >
4. > >
Which of the following is used to adsorb water?
1. Silica gel
2. Calcium acetate
3. Hairgel
4. Anhydrous Ca
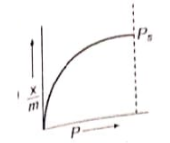
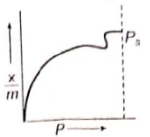
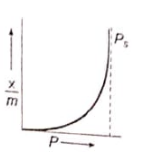
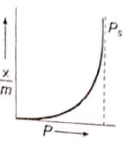
Which of the following adsorption isotherms represents the adsorption of a gas by a solid involving multilayers of formation? ( = saturation pressure)
1. 
2. 
3. 
4. 
A plot of log against log P for the adsorption of a gas on a solid gives a
straight line with slope equal to :
1.
2. n
3. log K
4. K
The function of zymase is to:
1. Change starch into sugar.
2. Ferment glucose to alcohol and CO2.
3. Change malt sugar into glucose.
4. Change starch into malt sugar and dextrin.
Surface tension of lyophilic sols is :
1. Lower than water.
2. More than water.
3. Equal to water.
4. None of the above.
All colloids:
1. Are suspension of one phase in another.
2. Are two-phase systems.
3. Contain only water-soluble particles.
4. Are true solutions.
Bredig’s arc method cannot be used for the preparation of colloidal sol of:
1. Copper
2. Gold
3. Silver
4. Sodium
Which of the following electrolyte will most effective in coagulation of negative sol?
1.
2.
3.
4.
The minimum amount of an electrolyte required to cause coagulation of a sol is called :
1. Coagulation value
2. Gold number
3. Protective value
4. None of these
Peptization involves:
1. Precipitation of colloidal particles.
2. Disintegration of colloidal aggregates.
3. Purification of colloids.
4. Both (1) and (3)
An emulsifying agent is a substance which
1. Stabilizes the emulsion.
2. De-stabilizes the emulsion.
3. Coagulates the emulsion.
4. Break the interfacial film between suspended particle and medium.
Colloidal solution of gold is prepared by :
1. Colloidal mill
2. Double decomposition method
3. Bredig’s method
4. Peptization
Artificial rain is caused by spraying:
1. Opposite charged colloidal dust over a cloud
2. Same charged colloidal dust over a cloud
3. Both
4. None of these
On adding AgN solution into Kl solution, a negatively charged colloidal sol is obtained when they are in :
1. 5O mL of O.1 M AgN+50 mL of 0.1M Kl
2. 5O mL of O.1 M AgN+5O mL of 0.2 M Kl
3. 5O mL of O.2 M AgN+5O mL of 0.1 M Kl
4. None of the above
The gold numbers of protective colloids A, B, C and D are 0.04, 0.004, 10 and 40
respectively. The protective powers of A, B, C and D are in the order :
1. A>B>C>D
2. B>A>C>D
3. D>C>A>B
4. D>C>B>A
The Tyndall effect associated with colloidal particles is due to :
1. Presence of electrical charges
2. Scattering of light
3. Absorption of light
4. Reflection of light
Blue colour of the sky is due to :
1. Absorption of light by dust particles.
2. Reflection of light by dust particles.
3. Scattering of light by dust particles.
4. None of the above.