One gm metal was discharged by the passage of 1.81 x electrons.
What is the atomic weight of metal?
1. 33.35
2. 133.4
3. 66.7
4. None of these
How much time is required for complete decomposition of 4 moles of water using 4 ampere?
1. 3.86 X sec
2. 1.93 X sec
3. 96500 sec
4. 48250 sec
If 0.50 L of a 0.60 M SnS solution is electrolyzed for a period of 30.0 min
using a current of 4.60 A. If inert electrodes are used, what is the final
concentration of remaining in the solution ? [at. Wt. of Sn = 119]
1. 0.342 M
2. 0.544 M
3. 0.389 M
4. 0.514 M
An electrolysis of a oxytungsten complex ion using 1.10 A for 40 min produces 0.838 g of tungsten. What is the charge on tungsten in the material ? (Atomic weight: W = 184)
1. 6
2. 2
3. 4
4. 1
Cadmium amalgam is prepared by electrolysis of a solution of Cd using a
mercury cathode. How long should a current 4 A be passed in order to prepare 10% by wt. Cd in Cd-Hg amalgam on cathode of 4.5 g Hg? (atomic weight of Cd = 112)
1. 400 sec
2. 215.40 sec
3. 861.6 sec
4. 430.8 sec
(s)| l (0.1 M) half cell ¡s connected to a (aq) | (1 bar) | Pt half cell and emf is found to be 0.7714 V. If = 0.535 V, find the pH of half cell.
1. 1
2. 3
3. 5
4. 7
If is 1.69 V and is 1.40 V, then will be :
1. 0.19 V
2. 2.945 V
3. 1.255 V
4. None of the above
Consider the following standard electrode potentials and calculate the
equilibrium constant at 25°C for the indicated disproportionation reaction :
;
;
1. 1.2 X
2. 2.4 X
3. 6.3 X
4. 1.5 X
Ionisation constant of a weak acid (HA) in terms of and is
1. =
2. =
3.=
4. None of these
Equivalent conductivity of is related to molar conductivity by the expression:
1. =
2. =
3. =
4. =
The limiting equivalent conductivity of NaCl, KCl and KBr are 126.5, 150.0 and
151.55 , respectively. The limiting equivalent ionic conductance for is 78 S . The limiting equivalent ionic conductance for ions would be :
1. 128
2. 125
3. 49
4. 50
The increase in equivalent conductance of a weak electrolyte with dilution is
due to
1. Increase in degree of dissociation and decrease ¡n ionic mobility
2. Decrease in degree of dissociation and decrease in ionic mobility
3. Increase in degree of dissociation and increase in ionic mobility
4. Decrease in degree of dissociation and increase in ionic mobility
The electric conduction of a salt solution in water depends on the:
1. Size of its molecules
2. Shape of its molecules
3. Size of solvent molecules
4. Extent of its ionization
A graph was plotted molar conductivity of various electrolytes (NaCl, HCl and
OH) and (in ). Correct setting :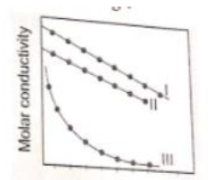
1. l(NaCl), ll(HCl), lll(OH)
2. l(HCl), ll(NaCl), lll(OH)
3. l(NOH), ll(NaCl), lll(HCl)
4. l(OH), ll(HCl), lll(NaCl)