When orthophosphoric acid (H₃PO₄) is heated, it undergoes dehydration to form different condensed phosphoric acids depending on the temperature.
Identify the correct products (X) and (Y) from the following scheme:
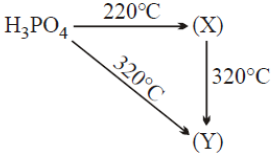
1. (X) = Phosphorous acid , (Y) = Metaphosphoric acid
2. (X) = Pyrophosphoric acid , (Y) = Metaphosphoric acid
3. (X) = Metaphosphoric acid , (Y) = Pyrophosphoric acid
4. (X) = Metaphosphoric acid , (Y) = Phosphene gas
Boron forms type of halides. The correct increasing order of Lewis-acid strength of these halides is
1. > > >
2. > > >
3. > > >
4. > > >
Which one of the following compounds on strong heating evolves ammonia gas?
1.
2.
3.
4.
The compound
1. Pyramidal and more basic than
2. Planar and less basic than
3. Pyramidal and less basic than
4. Planar and more basic than
Compound (A) is -
| 1. | A linear silicone | 2. | A chlorosilane |
| 3. | A linear silane | 4. | A network silane |
Match List - I with List - II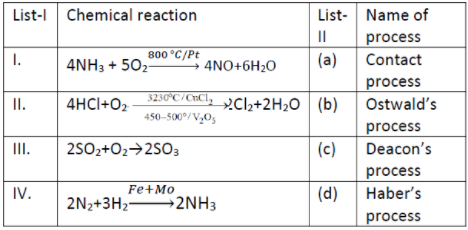
1. l-a, 11-b, III-d, IV-c
2. l-b, II-c, III-a, IV-d
3. l-a, II-d, III-C, IV-b
4. l-a, II-c, III-b, IV-d
Ammonia can be dried by
1. Conc.
2.
3. CaO
4. anhydrous
Which one of the following statements is not true regarding diborane?
1. It has two bridging hydrogens and four perpendiculars to the rest.
2. When methylated, the product is
3. The bridging hydrogens are in a plane perpendicular to the rest.
4. All the B-H bond distances are equal.
There is no S−S bond in
Borax is actually made of two tetrahedral and two triangular units joined together and should be written as:
Consider the following statements about borax:
(a) Each boron atom has four B-O bonds
(b) Each boron atom has three B-O bonds
(c) Two boron atoms have four B-0 bonds while the other two have three B-O bonds
(d) Each boron atom has one -OH groups
Select the correct statement(s);
1. a, b
2. b,c
3. c, d
4. a, c
What condition or substance is used to react with white phosphorus (P4)
![]() to selectively produce phosphorus trioxide (P2O3)?
to selectively produce phosphorus trioxide (P2O3)?
1. Dry
2. A mixture of and
3. Moist
4. in the presence of an aqueous NaO
Identify the correct order of solubility of , CuS, and ZnS in aqueous medium
1. CuS > ZnS >
2. ZnS > > CuS
3. > CuS > ZnS
4. > ZnS > CuS
is -
1. Monobasic acid and weak Lewis acid
2. Monobasic and weak Bronsted acid
3. Monobasic and strong Lewis acid
4. Tribasic and weak Bronsted acid
When is oxidised by in alkaline medium, converts into
1.
2.
3.
4.
This reaction is made to proceed in forward direction by -
1. Addition of Cis- 1,2 diol
2. Addition of borax
3. Addition of trans 1,2- diol
4. Addition of
A mixture of boric acid with ethyl alcohol burns with green edged flame due to the formation of -
1. Ethyl borax
2. Ethyl borate
3. Methyl borax
4. Methyl borate
Which of the following leaves no residue on heating?
1. Pb(NO3)2
2.
3. Cu(NO3)2
4.
Which amongst the following reactions
cannot be used for the preparation of the halogen acid?
An Aqueous solution of borax reacts with 2 moles of acid due to -
1. Formation of 2 moles of B(OH)3 only
2. Formation of 2 moles of [B(OH)4]- only
3. Formation of 1 mole each of B(OH)3 and [B(OH)4]-
4. Formation of 2 moles each of [B(OH)4]- and B(OH)3, only [B(OH)4]- reacts with an acid
The species present in solution when is dissolved in water are -
1.
2.
3.
4.
Which of the following is most easily hydrolysed amongst the following
1.
2.
3.
4.
Which of the following pair has bleaching property?
1.
2.
3.
4.
Nitrogen is liberated by the thermal decomposition of only
1.
2.
3.
4. All the three
Which of the following oxides of nitrogen is paramagnetic
1.
2.
3.
4.
In which of the following arrangement, the order is not according to the property indicated against it