Incorrect statement among the following is :
| 1. | Pv work is usually negligible for solid and liquid. |
| 2. | For a closed system with P-V work only, an isobaric process that has q= +ve must have \(\Delta \mathrm{T}=+\mathrm{ve}\). |
| 3. | For a cyclic process q= - w. |
| 4. | Black phosphorus is most stable form of P but \(\mathrm{H}_{\mathrm{f}}^{\circ}=0 \) for white phosphorus. |
Temperature and heat are:
1. Extensive properties
2. Intensive properties
3. Intensive and extensive properties respectively
4. Extensive and intensive properties respectively
In a flask, colourless NO(g) is in equilibrium with brown coloured NO(g) . At
equilibrium when the flask is heated to 100 C, the brown colour deepens and on
colling it becomes coloured. Which statement is incorrect about this observation?
1. The H for the reaction NO(g)2NO(g) is + ve
2. Paramagnetism increases on cooling
3. The H-U at 100 C is equal to 200 cal
4. Dimerisation is reduced on heating
The incorrect expression among the following is:
1. In isothermal process,
2.
3.
4.
Enthalpy of vaporisation for water is 186.5 kJ . The entropy change during vaporisation in kJ is -
(1) 0.5
(2) 1.0
(3) 1.5
(4) 2.0
The standard change is Gibbs energy for the reaction, is :
(1) 100kJ
(2) -90kJ
(3) 90kJ
(4)-100 kJ
For a reaction at enthalpy change (H) and entropy change (S) are and
respectively. The reaction is:
(1) Spontaneous
(2) Non-spontaneous
(3) Instantaneous
(4) None of the above
The entropy change for the reaction given below,
is ..... at 300 K. Standard entropies of and respectively.
(1)
(2)
(3)
(4) None of these
A Carnot engine operates between temperature T and 400 K (T > 400 K). If efficiency of engine is 25%, the temperature T is:
1. 400 K
2. 500 K
3. 533.3 K
4. 600 K
The work is done in an open vessel at 300 K, when 112 g irone reacts with dil. HCI is:
1. 1.2 kcal
2. 0.6 kcal
3. 0.2 kcal
4. 2 kcal
Boiling point of a liquid is 50 K at 1 atm and . what will be its b.p at 10 atm?
(1) 150 K
(2) 75 K
(3) 100 K
(4) 200 K
The internal energy change when a system goes from state A to B is 40 kJ/mol. If the system goes from A to B by a reversible path and returns to state A by an irreversible path and returns to state A by an irreversible path. What would be the net change in internal energy?
1. 40 kJ
2. >40 kJ
3. <40 kJ
4. Zero
At 1 atm pressure,
The temperature of the reaction at equilibrium is :
(1) 400 K
(2) 330 K
(3) 200K
(4) 110 K
(1) 1000K
(2) 1250 K
(3) 500 K
(4) 750 K
1.
2. R
3.
4.
Which plot represents an exothermic reaction?
| 1. |  |
2. |  |
| 3. |  |
4. |  |
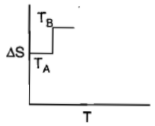
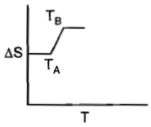
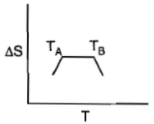
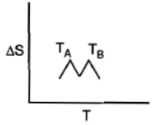
For a given substance, melting point and freezing point is , which of the following represents the correct variation of vs T?
1. 
2. 
3. 
4. 
Which of the following is correct for an ideal gas?
1.
2.
3.
4. All of these
The difference between heat of reaction at constant pressure and constant volume for the reaction given below at 25 in kJ is:
(1) -7.43
(2) +3.72
(3) -3.72
(4) +7.43
The value of for the reaction is -446kJ . If the ionisation energy of Cu(g) is 745 kJ and the electron affinity of I(g) is -295kJ , then the value of for the formation of one mole CuI(g) from Cu(g) and I(g) is:
(1) -446 kJ
(2) 450 kJ
(3) 594 kJ
(4) 4 kJ
Given enthalpy of formation of (g) and CaO(s) are -84.0kJ and -152kJ respectively and the enthalpy of the reaction,
is 42 kJ. The enthalpy of formation of (s) is:
1. -42 kJ
2. -202 kJ
3. +202 kJ
4. -288 kJ
The heat of combustion for C, and are -349.0, -241.8 and -906.7 kJ respectively. the heat of formation of is:
(1) 174.1 kJ
(2) 274.1 kJ
(3) 374.1 kJ
(4) 74.1 kJ