The Prussian blue colour obtained during the test of nitrogen by Lassaigne's test is due to the formation of-
| 1. | Fe4[Fe(CN)6]3 | 2. | Na3[Fe(CN)6] |
| 3. | Fe(CN)3 | 4. | Na4[Fe(CN)5NOS] |
In Kjeldahl's method of estimation of nitrogen, K2SO4 acts as:
| 1. | An oxidizing agent | 2. | A catalytic agent |
| 3. | A hydrolyzing agent | 4. | A boiling point elevator |
Soda extract is prepared by-
1. Fusing soda and mixture of hydrocarbons, and then extracted with water
2. Dissolving NaHCO3 and mixture of hydrocarbons in dil. HCl
3. Boiling Na2CO3 and mixture of hydrocarbons in dil. HCl
4. Boiling Na2CO3 and mixture of hydrocarbons in distilled water
A compound that does not give a positive test in Lassaigne’s test for nitrogen is:
1. Urea
2. Hydrazine
3. Azobenzene
4. Phenylhydrazine
In Kjeldahl's method for estimation of nitrogen present in a soil sample, ammonia evolved from 0.75 g of sample neutralized 10 mL of 1 M . The percentage of nitrogen in the soil is:
(1) 37.33
(2) 45.33
(3) 35.33
(4) 43.33
Ether and benzene can be separated by :-
(1) Filtration
(2) Distillation
(3) Crystallization
(4) Sublimation
The colour of the solution that gets formed by mixing sodium nitroprusside to an alkaline solution of sulfide ions, is-
| 1. | Red | 2. | Blue |
| 3. | Brown | 4. | Purple |
A strong base can abstract an -hydrogen from
1. alkene
2.amine
3. ketone
4. alkane
In Duma's method of estimation of nitrogen, 0.35 g of an organic compound gave 55 ml of nitrogen collected at 300 K temperature and 715 mm pressure. The percentage composition of nitrogen in the compound would be:
(Aqueous tension at 300 K = 15 mm)
1. 16.45
2. 27.45
3. 44.45
4. 35.45
Buta-1,3-diene and But-2-yne are:
1. Position isomers
2. Functional isomers
3. Chain isomers
4. Tautomers
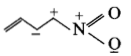
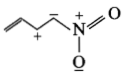
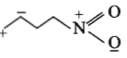
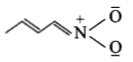
The least stable resonance structure among the following is:
1. 
2. 
3. 
4. 
0.26 g of an organic compound gave 0.039 g of water and 0.245 g of carbon dioxide on combustion. The percentage of C in the organic compound is-
1. 35%
2. 25%
3. 2%
4. 90%
In an estimation of sulphur by the carius method, 0.2175 g of the substance gave 0.5825 g of BaSO4 . The percentage composition of S in the compound is-
1. 66%
2. 20%
3. 37%
4.82%
0.284 g of an organic substance gave 0.287 g AgCl in a carius method for the estimation of halogen. The percentage of Cl in the compound is-
1. 5%
2. 18%
3. 25%
4. 33%
0.24 g of an organic compound containing phosphorous gave 0.66 g of Mg2P2O7 by the usual analysis. The percentage of phosphorous in the compound is-
1. 77%
2. 72%
3. 87%
4. 60 %
0.1688 g organic compound when analyzed by the Dumas method yields 31.7 mL of moist nitrogen measured at 14º C and 758 mm mercury pressure. The % of the nitrogen in the organic compound (Aqueous tension at 14 º C =12 mm) is:
1. 30.9%
2. 10%
3. 40%
4. 21.9 %
0.6 g of an organic compound was Kjeldahlised and NH3 evolved was absorbed into 50 mL of semi-normal solution of H2SO4. The residual acid solution was diluted with distilled water and the volume made up to 150 mL. 20 mL of this diluted solution required 35 mL of N/20 NaOH solution for complete neutralization.
1. 24.2%
2. 32.4%
3.27.6 %
4. 20.8%