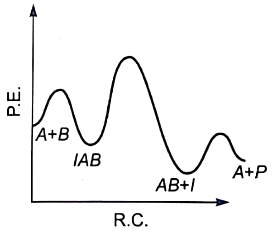
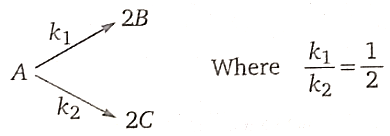1.
1.
2.
3.
4.
2.
|
Ex. No.
|
[A/M]
|
[B/M]
|
Initial rate
()
|
|
1.
|
0.01
|
0.01
|
|
|
2.
|
0.02
|
0.01
|
|
|
3.
|
0.02
|
0.01
|
|
1. M/sec
2. M/sec
3. M/sec
4. M/sec
3.
1.
2.
3.
4.
4.
1. 1 : 0.301
2. 0.125 : 0.602
3. 1 : 602
4. None of these
5.
1. 10 sec
2. 100 sec
3. 1000 sec
4. 434 sec
6.
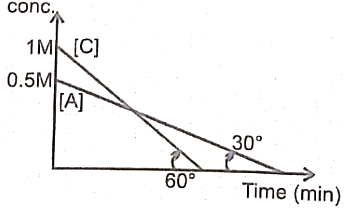
Which of the following is true:
a.
b.
c.
d.
1. a,b
2.a,b,c
3.c,d
4. a,d
7.
1. 0.0125 M
2. 0.025 M
3. 0.05 M
4. None of these
8.
1.
2.
3.
4.
9.
1.
2.
3.
4.
10. The gaseous decomposition reaction, A(g) 2B(g) + C(g) is observed to first order over the excess of liquid water at It is found that after 10 minutes the total pressure of the system is 188 torr and after a very long time, it is 388 torr. The rate constant of the reaction is :
[Given : vapour pressure of at is 28 torr (In 2 = 0.7, In 3 = 1.1, In 10 = 2.3)]
1. 0.02
2. 1.2
3. 0.2
4. None of these
11.
1. 135 min
2. 103.7 min
3. 38.7 min
4. 45 min
12.
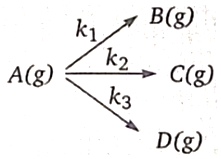
1. 0.288
2. 0.577
3. 1.154
4. None of these
13.
1. 1.25
2. 0.75
3. 1.50
4. 2.50
14. For the given hypothetical elementary parallel reaction,
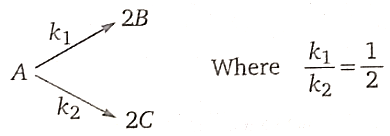
Initially, only 2 moles of A are present. The total no. of moles of A, B, and C at the end of 75% reaction are:
1. 2
2. 3
3. 4
4. 3.5
15. A hydrogenation reaction is carried out at 500 K. If the same reaction is carried out in presence of a catalyst at the same rate with same frequency factor, the temperature required is 400 K the activation energy of the reaction, if the catalyst lowers the activation energy barrier by 16 kJ/mol is:
1. 100 kJ/mol
2. 80 kJ/mol
3. 60 kJ/mol
4. None of th above
16.
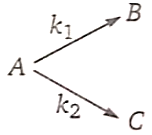
1. 100 kJ/mol
2. 120 kJ/mol
3. 116 kJ/mol
4. 220 kJ/mol
17.
1. 10
2. 20
3. 25
4. None of the above
18.
1.
2.
3.
4.
19.
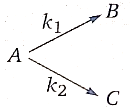
1. 757.48 K
2. 378.74 K
3. 600.91 K
4. None of the above
*If above link doesn't work, please go to test link from where you got the pdf and fill OMR from there
CLICK HERE to get FREE ACCESS for 2 days of ANY NEETprep course